Age-dependent poliovirus replication in the mouse central nervous system is determined by internal ribosome entry site-mediated translation
- PMID: 16501069
- PMCID: PMC1395422
- DOI: 10.1128/JVI.80.6.2589-2595.2006
Age-dependent poliovirus replication in the mouse central nervous system is determined by internal ribosome entry site-mediated translation
Abstract
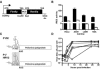
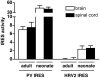
Mouse cells are not permissive for the replication of human rhinovirus type 2 (HRV2). To determine the role of the HRV2 internal ribosome entry site (IRES) in determining species specificity, a recombinant poliovirus (P1/HRV2) was constructed by substituting the poliovirus IRES with the IRES from HRV2. This recombinant virus replicated in all human and murine cell lines examined, demonstrating that the HRV2 IRES does not limit viral replication in transformed murine cells. P1/HRV2 replicated in the brain and spinal cord in neonatal but not adult mice transgenic for the poliovirus receptor, CD155. Passage of P1/HRV2 in mice led to selection of a virus that caused paralysis in neonatal mice. To determine the relationship between HRV2 IRES-mediated translation and replication of P1/HRV2 in mice, recombinant human adenoviruses were used to express bicistronic mRNAs in murine organs. The results demonstrate that the HRV2 IRES mediates translation in organs of neonatal but not adult mice. These findings show that HRV2 IRES-mediated translation is a determinant of virus replication in the murine brain and spinal cord and suggest that the IRES determines the species specificity of HRV2 infection.
Figures

Similar articles
-
Poliovirus tropism and attenuation are determined after internal ribosome entry.J Clin Invest. 2004 Jun;113(12):1743-53. doi: 10.1172/JCI21323. J Clin Invest. 2004. PMID: 15199409 Free PMC article.
-
Attenuation of rabies virus replication and virulence by picornavirus internal ribosome entry site elements.J Virol. 2009 Feb;83(4):1911-9. doi: 10.1128/JVI.02055-08. Epub 2008 Dec 10. J Virol. 2009. PMID: 19073737 Free PMC article.
-
Growth phenotypes and biosafety profiles in poliovirus-receptor transgenic mice of recombinant oncolytic polio/human rhinoviruses.J Med Virol. 2008 Feb;80(2):352-9. doi: 10.1002/jmv.21063. J Med Virol. 2008. PMID: 18098139
-
One hundred years of poliovirus pathogenesis.Virology. 2006 Jan 5;344(1):9-16. doi: 10.1016/j.virol.2005.09.015. Virology. 2006. PMID: 16364730 Review.
-
The pathogenesis of poliomyelitis: what we don't know.Adv Virus Res. 2008;71:1-50. doi: 10.1016/S0065-3527(08)00001-8. Adv Virus Res. 2008. PMID: 18585526 Review.
Cited by
-
Modification of the untranslated regions of human enterovirus 71 impairs growth in a cell-specific manner.J Virol. 2012 Jan;86(1):542-52. doi: 10.1128/JVI.00069-11. Epub 2011 Oct 26. J Virol. 2012. PMID: 22031931 Free PMC article.
-
Enterovirus infections of the central nervous system.Virology. 2011 Mar 15;411(2):288-305. doi: 10.1016/j.virol.2010.12.014. Epub 2011 Jan 20. Virology. 2011. PMID: 21251690 Free PMC article. Review.
-
A new animal model containing human SCARB2 and lacking stat-1 is highly susceptible to EV71.Sci Rep. 2016 Aug 8;6:31151. doi: 10.1038/srep31151. Sci Rep. 2016. PMID: 27499235 Free PMC article.
-
A host-specific, temperature-sensitive translation defect determines the attenuation phenotype of a human rhinovirus/poliovirus chimera, PV1(RIPO).J Virol. 2011 Jul;85(14):7225-35. doi: 10.1128/JVI.01804-09. Epub 2011 May 11. J Virol. 2011. PMID: 21561914 Free PMC article.
-
Immunodeficient mouse models with different disease profiles by in vivo infection with the same clinical isolate of enterovirus 71.J Virol. 2014 Nov;88(21):12485-99. doi: 10.1128/JVI.00692-14. Epub 2014 Aug 20. J Virol. 2014. PMID: 25142603 Free PMC article.
References
-
- Arruda, E., T. R. Boyle, B. Winther, D. C. Pevear, J. M. Gwaltney, Jr., and F. G. Hayden. 1995. Localization of human rhinovirus replication in the upper respiratory tract by in situ hybridization. J. Infect. Dis. 171:1329-1333. - PubMed
-
- Bardin, P. G., S. L. Johnston, G. Sanderson, B. S. Robinson, M. A. Pickett, D. J. Fraenkel, and S. T. Holgate. 1994. Detection of rhinovirus infection of the nasal mucosa by oligonucleotide in situ hybridization. Am. J. Respir. Cell Mol. Biol. 10:207-213. - PubMed
-
- Borman, A., and R. J. Jackson. 1992. Initiation of translation of human rhinovirus RNA: mapping the internal ribosome entry site. Virology 188:685-696. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

