FUS1 regulates the opening and expansion of fusion pores between mating yeast
- PMID: 16495338
- PMCID: PMC1446097
- DOI: 10.1091/mbc.e05-11-1015
FUS1 regulates the opening and expansion of fusion pores between mating yeast
Abstract
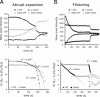
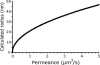
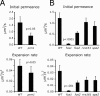
Mating yeast cells provide a genetically accessible system for the study of cell fusion. The dynamics of fusion pores between yeast cells were analyzed by following the exchange of fluorescent markers between fusion partners. Upon plasma membrane fusion, cytoplasmic GFP and DsRed diffuse between cells at rates proportional to the size of the fusion pore. GFP permeance measurements reveal that a typical fusion pore opens with a burst and then gradually expands. In some mating pairs, a sudden increase in GFP permeance was found, consistent with the opening of a second pore. In contrast, other fusion pores closed after permitting a limited amount of cytoplasmic exchange. Deletion of FUS1 from both mating partners caused a >10-fold reduction in the initial permeance and expansion rate of the fusion pore. Although fus1 mating pairs also have a defect in degrading the cell wall that separates mating partners before plasma membrane fusion, other cell fusion mutants with cell wall remodeling defects had more modest effects on fusion pore permeance. Karyogamy is delayed by >1 h in fus1 mating pairs, possibly as a consequence of retarded fusion pore expansion.
Figures


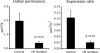

Similar articles
-
The Golgi-resident protease Kex2 acts in conjunction with Prm1 to facilitate cell fusion during yeast mating.J Cell Biol. 2007 Jan 15;176(2):209-22. doi: 10.1083/jcb.200609182. Epub 2007 Jan 8. J Cell Biol. 2007. PMID: 17210951 Free PMC article.
-
The plasma membrane proteins Prm1 and Fig1 ascertain fidelity of membrane fusion during yeast mating.Mol Biol Cell. 2007 Feb;18(2):547-56. doi: 10.1091/mbc.e06-09-0776. Epub 2006 Dec 6. Mol Biol Cell. 2007. PMID: 17151357 Free PMC article.
-
Prm1 prevents contact-dependent lysis of yeast mating pairs.Eukaryot Cell. 2004 Dec;3(6):1664-73. doi: 10.1128/EC.3.6.1664-1673.2004. Eukaryot Cell. 2004. PMID: 15590839 Free PMC article.
-
Membrane tension and membrane fusion.Curr Opin Struct Biol. 2015 Aug;33:61-7. doi: 10.1016/j.sbi.2015.07.010. Epub 2015 Aug 15. Curr Opin Struct Biol. 2015. PMID: 26282924 Free PMC article. Review.
-
Fusion pores and fusion machines in Ca2+-triggered exocytosis.Annu Rev Biophys Biomol Struct. 2006;35:135-60. doi: 10.1146/annurev.biophys.35.040405.101958. Annu Rev Biophys Biomol Struct. 2006. PMID: 16689631 Review.
Cited by
-
Nuclear fusion during yeast mating occurs by a three-step pathway.J Cell Biol. 2007 Nov 19;179(4):659-70. doi: 10.1083/jcb.200706151. J Cell Biol. 2007. PMID: 18025302 Free PMC article.
-
The Golgi-resident protease Kex2 acts in conjunction with Prm1 to facilitate cell fusion during yeast mating.J Cell Biol. 2007 Jan 15;176(2):209-22. doi: 10.1083/jcb.200609182. Epub 2007 Jan 8. J Cell Biol. 2007. PMID: 17210951 Free PMC article.
-
Comparative live-cell imaging analyses of SPA-2, BUD-6 and BNI-1 in Neurospora crassa reveal novel features of the filamentous fungal polarisome.PLoS One. 2012;7(1):e30372. doi: 10.1371/journal.pone.0030372. Epub 2012 Jan 24. PLoS One. 2012. PMID: 22291944 Free PMC article.
-
Prm1 targeting to contact sites enhances fusion during mating in Saccharomyces cerevisiae.Eukaryot Cell. 2010 Oct;9(10):1538-48. doi: 10.1128/EC.00116-10. Epub 2010 Aug 20. Eukaryot Cell. 2010. PMID: 20729291 Free PMC article.
-
Ergosterol promotes pheromone signaling and plasma membrane fusion in mating yeast.J Cell Biol. 2008 Feb 25;180(4):813-26. doi: 10.1083/jcb.200705076. J Cell Biol. 2008. PMID: 18299351 Free PMC article.
References
-
- Albillos, A., Dernick, G., Horstmann, H., Almers, W., Alvarez de Toledo, G., and Lindau, M. (1997). The exocytotic event in chromaffin cells revealed by patch amperometry. Nature 389, 509-512. - PubMed
-
- Alvarez-Dolado, M., Pardal, R., Garcia-Verdugo, J. M., Fike, J. R., Lee, H. O., Pfeffer, K., Lois, C., Morrison, S. J., and Alvarez-Buylla, A. (2003). Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425, 968-973. - PubMed
-
- Aravanis, A. M., Pyle, J. L., and Tsien, R. W. (2003). Single synaptic vesicles fusing transiently and successively without loss of identity. Nature 423, 643-647. - PubMed
-
- Archer, D. A., Graham, M. E., and Burgoyne, R. D. (2002). Complexin regulates the closure of the fusion pore during regulated vesicle exocytosis. J. Biol. Chem. 277, 18249-18252. - PubMed
-
- Atkinson, M. M., and Sheridan, J. D. (1988). Altered junctional permeability between cells transformed by v-ras, v-mos, or v-src. Am. J. Physiol. 255, C674-C683. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

