Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury
- PMID: 16478805
- PMCID: PMC1413773
- DOI: 10.1073/pnas.0506854103
Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury
Abstract
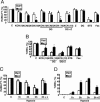
Ischemia-reperfusion (IR) injury induces endoplasmic reticulum (ER) stress and cell death. Bax Inhibitor-1 (BI-1) is an evolutionarily conserved ER protein that suppresses cell death and that is abundantly expressed in both liver and kidney. We explored the role of BI-1 in protection from ER stress and IR injury by using bi-1 knockout mice, employing models of transient hepatic or renal artery occlusion. Compared to wild-type bi-1 mice, bi-1 knockout mice subjected to hepatic IR injury exhibited these characteristics: (i) increased histological injury; (ii) increased serum transaminases, indicative of more hepatocyte death; (iii) increased percentages of TUNEL-positive hepatocytes; (iv) greater elevations in caspase activity; and (v) more activation of ER stress proteins inositol-requiring enzyme 1 and activating transcription factor 6 and greater increases in expression of ER stress proteins C/EBP homologous protein and spliced XBP-1 protein. Moreover, hepatic IR injury induced elevations in bi-1 mRNA in wild-type liver, suggesting a need for bi-1 gene induction to limit tissue injury. Similar sensitization of kidney to ER stress and IR injury was observed in bi-1(-/-) mice. We conclude that bi-1 provides endogenous protection of liver and kidney from ER stress and IR injury. Analysis of components of the bi-1-dependent pathway for protection from IR injury may therefore reveal new strategies for organ preservation.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


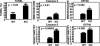

Similar articles
-
Sodium 4-phenylbutyrate protects against liver ischemia reperfusion injury by inhibition of endoplasmic reticulum-stress mediated apoptosis.Surgery. 2005 Aug;138(2):342-51. doi: 10.1016/j.surg.2005.04.019. Surgery. 2005. PMID: 16153446
-
BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress.Mol Cell. 2004 Aug 13;15(3):355-66. doi: 10.1016/j.molcel.2004.06.038. Mol Cell. 2004. PMID: 15304216
-
CAAT/enhancer binding protein-homologous protein deficiency attenuates liver ischemia/reperfusion injury in mice.Liver Transpl. 2018 May;24(5):645-654. doi: 10.1002/lt.25053. Epub 2018 Apr 16. Liver Transpl. 2018. PMID: 29524333
-
Decoding cell death signals in liver inflammation.J Hepatol. 2013 Sep;59(3):583-94. doi: 10.1016/j.jhep.2013.03.033. Epub 2013 Apr 6. J Hepatol. 2013. PMID: 23567086 Review.
-
Relevance of Endoplasmic Reticulum Stress Cell Signaling in Liver Cold Ischemia Reperfusion Injury.Int J Mol Sci. 2016 May 25;17(6):807. doi: 10.3390/ijms17060807. Int J Mol Sci. 2016. PMID: 27231901 Free PMC article. Review.
Cited by
-
Downregulation of XBP1 protects kidney against ischemia-reperfusion injury via suppressing HRD1-mediated NRF2 ubiquitylation.Cell Death Discov. 2021 Mar 2;7(1):44. doi: 10.1038/s41420-021-00425-z. Cell Death Discov. 2021. PMID: 33654072 Free PMC article.
-
Induction of heme oxygenase-1 protects mouse liver from apoptotic ischemia/reperfusion injury.Apoptosis. 2013 May;18(5):547-55. doi: 10.1007/s10495-013-0814-x. Apoptosis. 2013. PMID: 23435964 Free PMC article.
-
Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications.FEBS J. 2019 Jan;286(2):241-278. doi: 10.1111/febs.14608. Epub 2018 Aug 4. FEBS J. 2019. PMID: 30027602 Free PMC article. Review.
-
Control of lysosomal-mediated cell death by the pH-dependent calcium channel RECS1.Sci Adv. 2021 Nov 12;7(46):eabe5469. doi: 10.1126/sciadv.abe5469. Epub 2021 Nov 12. Sci Adv. 2021. PMID: 34767445 Free PMC article.
-
ATF6 mediates a pro-inflammatory synergy between ER stress and TLR activation in the pathogenesis of liver ischemia-reperfusion injury.Am J Transplant. 2014 Jul;14(7):1552-61. doi: 10.1111/ajt.12711. Epub 2014 Jun 5. Am J Transplant. 2014. PMID: 24903305 Free PMC article.
References
-
- Fondevila C., Busuttil R. W., Kupiec-Weglinski J. W. Exp. Mol. Pathol. 2003;74:86–93. - PubMed
-
- Cursio R., Gugenheim J., Ricci J. E., Crenesse D., Rostagno P., Maulon L., Saint-Paul M. C., Ferrua B., Auberger A. P. FASEB J. 1999;13:253–261. - PubMed
-
- Orrenius S., Zhivotovsky B., Nicotera P. Nat. Rev. Mol. Cell Biol. 2003;4:552–565. - PubMed
-
- Breckenridge D. G., Germain M., Mathai J. P., Nguyen M., Shore G. C. Oncogene. 2003;22:8608–8618. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

