Matrix protein mutant of vesicular stomatitis virus stimulates maturation of myeloid dendritic cells
- PMID: 16474127
- PMCID: PMC1395366
- DOI: 10.1128/JVI.80.5.2194-2205.2006
Matrix protein mutant of vesicular stomatitis virus stimulates maturation of myeloid dendritic cells
Abstract
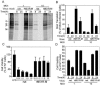
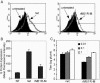
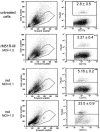
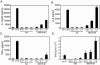
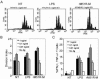
Matrix (M) protein mutants of vesicular stomatitis virus have recently been used as oncolytic viruses for tumor therapies and are being developed as vaccine vectors for heterologous antigens. Because dendritic cell (DC) maturation is an important correlate of tumor immunosurveillance and vaccine efficacy, we sought to determine the ability of a recombinant M protein mutant virus (rM51R-M virus) to mature DC in vitro. We have previously shown that rM51R-M virus is defective at inhibiting host gene expression in several cell lines compared to its recombinant wild-type counterpart, rwt virus. Therefore, rM51R-M virus allows the expression of genes involved in antiviral responses, such as the type I interferon (IFN) gene. Our results demonstrate that, in contrast to the rwt virus, rM51R-M virus induced the maturation of myeloid DC (mDC) populations, as indicated by an increase in the surface expression of CD40, CD80, and CD86 as well as the secretion of interleukin-12 (IL-12), IL-6, and type I IFN. In addition, mDC infected with rM51R-M virus effectively activated naïve T cells in vitro, whereas rwt virus-infected mDC were defective in antigen presentation. The inability of rwt virus to induce mDC maturation was correlated with the inhibition of host gene expression in rwt virus-infected cells. Our studies also indicated that the production of costimulatory molecules on mDC by rM51R-M virus was dependent on the type I IFN receptor, while maturation induced by this virus was largely independent of MyD88. These data indicate that rM51R-M virus effectively stimulates the maturation of mDC and has the potential to promote effective T-cell responses to vector-expressed antigens, activate DC at tumor sites during therapy, and aid in tumor immunosurveillance and destruction.
Figures
Similar articles
-
Stimulation of human dendritic cells by wild-type and M protein mutant vesicular stomatitis viruses engineered to express bacterial flagellin.J Virol. 2010 Nov;84(22):12093-8. doi: 10.1128/JVI.00406-10. Epub 2010 Sep 15. J Virol. 2010. PMID: 20844045 Free PMC article.
-
Vesicular stomatitis virus M protein mutant stimulates maturation of Toll-like receptor 7 (TLR7)-positive dendritic cells through TLR-dependent and -independent mechanisms.J Virol. 2009 Apr;83(7):2962-75. doi: 10.1128/JVI.02030-08. Epub 2009 Jan 14. J Virol. 2009. PMID: 19144711 Free PMC article.
-
Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses.Virology. 2004 Dec 5;330(1):34-49. doi: 10.1016/j.virol.2004.08.039. Virology. 2004. PMID: 15527832
-
Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus.J Virol. 2001 Dec;75(24):12169-81. doi: 10.1128/JVI.75.24.12169-12181.2001. J Virol. 2001. PMID: 11711608 Free PMC article.
-
Porcine monocyte-derived dendritic cells can be differentially activated by vesicular stomatitis virus and its matrix protein mutants.Vet Microbiol. 2018 Jun;219:30-39. doi: 10.1016/j.vetmic.2018.04.011. Epub 2018 Apr 8. Vet Microbiol. 2018. PMID: 29778202
Cited by
-
Impact of the Type I Interferon Receptor on the Global Gene Expression Program During the Course of Dendritic Cell Maturation Induced by Polyinosinic Polycytidylic Acid.J Interferon Cytokine Res. 2016 Jun;36(6):382-400. doi: 10.1089/jir.2014.0150. Epub 2016 Apr 1. J Interferon Cytokine Res. 2016. PMID: 27035059 Free PMC article.
-
Interplay between innate immunity and negative-strand RNA viruses: towards a rational model.Microbiol Mol Biol Rev. 2011 Sep;75(3):468-90, second page of table of contents. doi: 10.1128/MMBR.00007-11. Microbiol Mol Biol Rev. 2011. PMID: 21885681 Free PMC article. Review.
-
Vesicular stomatitis virus oncolytic treatment interferes with tumor-associated dendritic cell functions and abrogates tumor antigen presentation.J Virol. 2011 Dec;85(23):12160-9. doi: 10.1128/JVI.05703-11. Epub 2011 Sep 14. J Virol. 2011. PMID: 21917977 Free PMC article.
-
Oncolytic viruses: A novel treatment strategy for breast cancer.Genes Dis. 2021 Dec 16;10(2):430-446. doi: 10.1016/j.gendis.2021.11.011. eCollection 2023 Mar. Genes Dis. 2021. PMID: 37223527 Free PMC article. Review.
-
Stimulation of human dendritic cells by wild-type and M protein mutant vesicular stomatitis viruses engineered to express bacterial flagellin.J Virol. 2010 Nov;84(22):12093-8. doi: 10.1128/JVI.00406-10. Epub 2010 Sep 15. J Virol. 2010. PMID: 20844045 Free PMC article.
References
-
- Ahmed, M., S. D. Cramer, and D. S. Lyles. 2004. Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology 330:34-49. - PubMed
-
- Ahmed, M., M. O. McKenzie, S. Puckett, M. Hojnacki, L. Poliquin, and D. S. Lyles. 2003. Ability of M protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J. Virol. 77:4646-4657. - PMC - PubMed
-
- Bachmann, M. F., T. M. Kundig, G. Freer, Y. Li, C. Y. Kang, D. H. Bishop, H. Hengarner, and R. M. Zinkernagel. 1994. Induction of protective cytotoxic T cells with viral proteins. Eur. J. Immunol. 24:2228-2236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

