Intracellular assembly and budding of the Murine Leukemia Virus in infected cells
- PMID: 16472393
- PMCID: PMC1434767
- DOI: 10.1186/1742-4690-3-12
Intracellular assembly and budding of the Murine Leukemia Virus in infected cells
Abstract
Background: Murine Leukemia Virus (MLV) assembly has been long thought to occur exclusively at the plasma membrane. Current models of retroviral particle assembly describe the recruitment of the host vacuolar protein sorting machinery to the cell surface to induce the budding of new particles. Previous fluorescence microscopy study reported the vesicular traffic of the MLV components (Gag, Env and RNA). Here, electron microscopy (EM) associated with immunolabeling approaches were used to go deeply into the assembly of the "prototypic" MLV in chronically infected NIH3T3 cells.
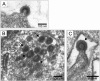
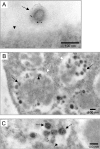
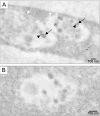
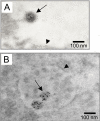
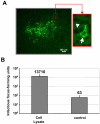
Results: Beside the virus budding events seen at the cell surface of infected cells, we observed that intracellular budding events could also occur inside the intracellular vacuoles in which many VLPs accumulated. EM in situ hybridization and immunolabeling analyses confirmed that these latter were MLV particles. Similar intracellular particles were detected in cells expressing MLV Gag alone. Compartments containing the MLV particles were identified as late endosomes using Lamp1 endosomal/lysosomal marker and BSA-gold pulse-chase experiments. In addition, infectious activity was detected in lysates of infected cells.
Conclusion: Altogether, our results showed that assembly of MLV could occur in part in intracellular compartments of infected murine cells and participate in the production of infectious viruses. These observations suggested that MLV budding could present similarities with the particular intracellular budding of HIV in infected macrophages.
Figures


Similar articles
-
Intracellular trafficking of Gag and Env proteins and their interactions modulate pseudotyping of retroviruses.J Virol. 2004 Jul;78(13):7153-64. doi: 10.1128/JVI.78.13.7153-7164.2004. J Virol. 2004. PMID: 15194792 Free PMC article.
-
Infectious HIV-1 assembles in late endosomes in primary macrophages.J Cell Biol. 2003 Aug 4;162(3):443-55. doi: 10.1083/jcb.200304008. Epub 2003 Jul 28. J Cell Biol. 2003. PMID: 12885763 Free PMC article.
-
Assembly of infectious HIV-1 in human epithelial and T-lymphoblastic cell lines.J Mol Biol. 2006 Jun 16;359(4):848-62. doi: 10.1016/j.jmb.2006.04.017. Epub 2006 Apr 25. J Mol Biol. 2006. PMID: 16682056
-
Cell types in the central nervous system infected by murine retroviruses: implications for the mechanisms of neurodegeneration.Histol Histopathol. 1994 Oct;9(4):845-58. Histol Histopathol. 1994. PMID: 7894152 Review.
-
Filovirus assembly and budding.Virology. 2006 Jan 5;344(1):64-70. doi: 10.1016/j.virol.2005.09.018. Virology. 2006. PMID: 16364737 Review.
Cited by
-
Equine Infectious Anemia Virus Gag Assembly and Export Are Directed by Matrix Protein through trans-Golgi Networks and Cellular Vesicles.J Virol. 2015 Dec 4;90(4):1824-38. doi: 10.1128/JVI.02814-15. Print 2016 Feb 15. J Virol. 2015. PMID: 26637458 Free PMC article.
-
The Delta variant SARS-CoV-2 spike protein uniquely promotes aggregation of pseudotyped viral particles.bioRxiv [Preprint]. 2022 Apr 8:2022.04.07.487415. doi: 10.1101/2022.04.07.487415. bioRxiv. 2022. Update in: Viruses. 2022 May 11;14(5):1024. doi: 10.3390/v14051024. PMID: 35441171 Free PMC article. Updated. Preprint.
-
A single residue substitution in the receptor-binding domain of H5N1 hemagglutinin is critical for packaging into pseudotyped lentiviral particles.PLoS One. 2012;7(11):e43596. doi: 10.1371/journal.pone.0043596. Epub 2012 Nov 2. PLoS One. 2012. PMID: 23133587 Free PMC article.
-
Multiple Gag domains contribute to selective recruitment of murine leukemia virus (MLV) Env to MLV virions.J Virol. 2013 Feb;87(3):1518-27. doi: 10.1128/JVI.02604-12. Epub 2012 Nov 14. J Virol. 2013. PMID: 23152533 Free PMC article.
-
Selective Isolation of Retroviruses from Extracellular Vesicles by Intact Virion Immunoprecipitation.Bio Protoc. 2018 Sep 5;8(17):e3005. doi: 10.21769/BioProtoc.3005. eCollection 2018 Sep 5. Bio Protoc. 2018. PMID: 34395797 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

