Tuning and playing a motor rhythm: how metabotropic glutamate receptors orchestrate generation of motor patterns in the mammalian central nervous system
- PMID: 16469790
- PMCID: PMC1779665
- DOI: 10.1113/jphysiol.2005.100610
Tuning and playing a motor rhythm: how metabotropic glutamate receptors orchestrate generation of motor patterns in the mammalian central nervous system
Abstract
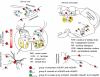
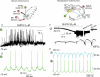
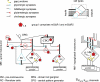
Repeated motor activities like locomotion, mastication and respiration need rhythmic discharges of functionally connected neurons termed central pattern generators (CPGs) that cyclically activate motoneurons even in the absence of descending commands from higher centres. For motor pattern generation, CPGs require integration of multiple processes including activation of ion channels and transmitter receptors at strategic locations within motor networks. One emerging mechanism is activation of glutamate metabotropic receptors (mGluRs) belonging to group I, while group II and III mGluRs appear to play an inhibitory function on sensory inputs. Group I mGluRs generate neuronal membrane depolarization with input resistance increase and rapid fluctuations in intracellular Ca(2+), leading to enhanced excitability and rhythmicity. While synchronicity is probably due to modulation of inhibitory synaptic transmission, these oscillations occurring in coincidence with strong afferent stimuli or application of excitatory agents can trigger locomotor-like patterns. Hence, mGluR-sensitive spinal oscillators play a role in accessory networks for locomotor CPG activation. In brainstem networks supplying tongue muscle motoneurons, group I receptors facilitate excitatory synaptic inputs and evoke synchronous oscillations which stabilize motoneuron firing at regular, low frequency necessary for rhythmic tongue contractions. In this case, synchronicity depends on the strong electrical coupling amongst motoneurons rather than inhibitory transmission, while cyclic activation of K(ATP) conductances sets its periodicity. Activation of mGluRs is therefore a powerful strategy to trigger and recruit patterned discharges of motoneurons.
Figures

Similar articles
-
Fictive locomotor patterns generated by tetraethylammonium application to the neonatal rat spinal cord in vitro.Neuroscience. 2006;137(2):659-70. doi: 10.1016/j.neuroscience.2005.09.025. Epub 2005 Nov 14. Neuroscience. 2006. PMID: 16289841
-
Role of group II and III metabotropic glutamate receptors in rhythmic patterns of the neonatal rat spinal cord in vitro.Exp Brain Res. 2004 Jun;156(4):495-504. doi: 10.1007/s00221-003-1798-5. Epub 2004 Mar 9. Exp Brain Res. 2004. PMID: 15007577
-
[The role of group II and III metabotropic glutamate receptors in modulation of miniature synaptic activity in frog spinal cord motoneurons].Tsitologiia. 2008;50(9):747-56. Tsitologiia. 2008. PMID: 18959186 Russian.
-
Reorganization of the human central nervous system.Gen Physiol Biophys. 2000 Oct;19 Suppl 1:11-240. Gen Physiol Biophys. 2000. PMID: 11252267 Review.
-
Excitatory components of the mammalian locomotor CPG.Brain Res Rev. 2008 Jan;57(1):56-63. doi: 10.1016/j.brainresrev.2007.07.002. Epub 2007 Nov 7. Brain Res Rev. 2008. PMID: 17988744 Review.
Cited by
-
Nicotinic receptor activation contrasts pathophysiological bursting and neurodegeneration evoked by glutamate uptake block on rat hypoglossal motoneurons.J Physiol. 2016 Nov 15;594(22):6777-6798. doi: 10.1113/JP272591. Epub 2016 Aug 3. J Physiol. 2016. PMID: 27374167 Free PMC article.
-
Microelectrode arrays in combination with in vitro models of spinal cord injury as tools to investigate pathological changes in network activity: facts and promises.Front Neuroeng. 2013 Mar 4;6:2. doi: 10.3389/fneng.2013.00002. eCollection 2013. Front Neuroeng. 2013. PMID: 23459694 Free PMC article.
-
Measured motion: searching for simplicity in spinal locomotor networks.Curr Opin Neurobiol. 2009 Dec;19(6):572-86. doi: 10.1016/j.conb.2009.10.011. Epub 2009 Nov 10. Curr Opin Neurobiol. 2009. PMID: 19896834 Free PMC article. Review.
-
Cerebellar cortical output encodes temporal aspects of rhythmic licking movements and is necessary for normal licking frequency.Eur J Neurosci. 2010 Jul;32(1):41-52. doi: 10.1111/j.1460-9568.2010.07244.x. Epub 2010 Jun 28. Eur J Neurosci. 2010. PMID: 20597972 Free PMC article.
-
Emerging Roles of Filopodia and Dendritic Spines in Motoneuron Plasticity during Development and Disease.Neural Plast. 2016;2016:3423267. doi: 10.1155/2016/3423267. Epub 2015 Dec 30. Neural Plast. 2016. PMID: 26843990 Free PMC article. Review.
References
-
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. - PubMed
-
- Alford S, Schwartz E, Di Prisco GV. The pharmacology of vertebrate spinal central pattern generators. Neuroscientist. 2003;9:217–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

