Roles of protease-activated receptors in a mouse model of endotoxemia
- PMID: 16434493
- PMCID: PMC1895289
- DOI: 10.1182/blood-2005-08-3130
Roles of protease-activated receptors in a mouse model of endotoxemia
Abstract
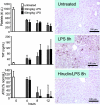
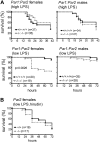
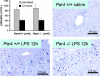
Endotoxemia is often associated with extreme inflammatory responses and disseminated intravascular coagulation. Protease-activated receptors (PARs) mediate cellular responses to coagulation proteases, including platelet activation and endothelial cell reactions predicted to promote inflammation. These observations suggested that PAR activation by coagulation proteases generated in the setting of endotoxemia might promote platelet activation, leukocyte-mediated endothelial injury, tissue damage, and death. Toward testing these hypotheses, we examined the effect of PAR deficiencies that ablate platelet and endothelial activation by coagulation proteases in a mouse endotoxemia model. Although coagulation was activated as measured by thrombin-antithrombin (TAT) production and antithrombin III (ATIII) depletion, Par1(-/-), Par2(-/-), Par4(-/-), Par2(-/-):Par4(-/-), and Par1(-/-):Par2(-/-) mice all failed to show improved survival or decreased cytokine responses after endotoxin challenge compared with wild type. Thus, our results fail to support a necessary role for PARs in linking coagulation to inflammation or death in this model. Interestingly, endotoxin-induced thrombocytopenia was not diminished in Par4(-/-) mice. Thus, a mechanism independent of platelet activation by thrombin was sufficient to cause thrombocytopenia in our model. These results raise the possibility that decreases in platelet count in the setting of sepsis may not be caused by disseminated intravascular coagulation but instead report on a sometimes parallel but independent process.
Figures
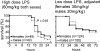


Similar articles
-
Genetic evidence that protease-activated receptors mediate factor Xa signaling in endothelial cells.J Biol Chem. 2002 May 3;277(18):16081-7. doi: 10.1074/jbc.M108555200. Epub 2002 Feb 15. J Biol Chem. 2002. PMID: 11850418
-
Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa.Proc Natl Acad Sci U S A. 2000 May 9;97(10):5255-60. doi: 10.1073/pnas.97.10.5255. Proc Natl Acad Sci U S A. 2000. PMID: 10805786 Free PMC article.
-
Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia.Blood. 2004 Feb 15;103(4):1342-7. doi: 10.1182/blood-2003-09-3051. Epub 2003 Oct 23. Blood. 2004. PMID: 14576054 Free PMC article.
-
Tissue factor, coagulation proteases, and protease-activated receptors in endotoxemia and sepsis.Crit Care Med. 2004 May;32(5 Suppl):S293-7. doi: 10.1097/01.ccm.0000128445.95144.b8. Crit Care Med. 2004. PMID: 15118533 Review.
-
Roles of Coagulation Proteases and PARs (Protease-Activated Receptors) in Mouse Models of Inflammatory Diseases.Arterioscler Thromb Vasc Biol. 2019 Jan;39(1):13-24. doi: 10.1161/ATVBAHA.118.311655. Arterioscler Thromb Vasc Biol. 2019. PMID: 30580574 Free PMC article. Review.
Cited by
-
1-Piperidine Propionic Acid Protects from Septic Shock Through Protease Receptor 2 Inhibition.Int J Mol Sci. 2024 Oct 30;25(21):11662. doi: 10.3390/ijms252111662. Int J Mol Sci. 2024. PMID: 39519216 Free PMC article.
-
Protease-activated receptors in health and disease.Physiol Rev. 2023 Jan 1;103(1):717-785. doi: 10.1152/physrev.00044.2021. Epub 2022 Jul 28. Physiol Rev. 2023. PMID: 35901239 Free PMC article. Review.
-
Procoagulant signalling mechanisms in lung inflammation and fibrosis: novel opportunities for pharmacological intervention?Br J Pharmacol. 2008 Mar;153 Suppl 1(Suppl 1):S367-78. doi: 10.1038/sj.bjp.0707603. Epub 2008 Jan 28. Br J Pharmacol. 2008. PMID: 18223674 Free PMC article. Review.
-
FoxM1 regulates re-annealing of endothelial adherens junctions through transcriptional control of beta-catenin expression.J Exp Med. 2010 Aug 2;207(8):1675-85. doi: 10.1084/jem.20091857. Epub 2010 Jul 26. J Exp Med. 2010. PMID: 20660612 Free PMC article.
-
Protease-activated receptor-2 augments experimental crescentic glomerulonephritis.Am J Pathol. 2007 Sep;171(3):800-8. doi: 10.2353/ajpath.2007.061155. Epub 2007 Jul 19. Am J Pathol. 2007. PMID: 17640968 Free PMC article.
References
-
- Esmon CT, Fukudome K, Mather T, et al. Inflammation, sepsis, and coagulation. Haematologica. 1999;84: 254-259. - PubMed
-
- Camerer E, Kolsto AB, Prydz H. Cell biology of tissue factor, the principal initiator of blood coagulation. Thromb Res. 1996;81: 1-41. - PubMed
-
- Esmon CT. Sepsis. A myriad of responses. Lancet. 2001;358(suppl): S61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

