Traumatic injury and the presence of antigen differentially contribute to T-cell recruitment in the CNS
- PMID: 16421293
- PMCID: PMC6675378
- DOI: 10.1523/JNEUROSCI.3502-05.2006
Traumatic injury and the presence of antigen differentially contribute to T-cell recruitment in the CNS
Abstract
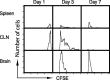
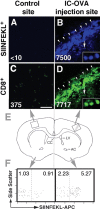
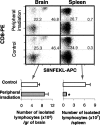
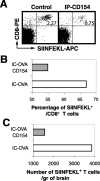
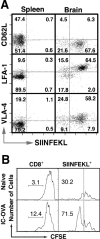
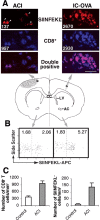
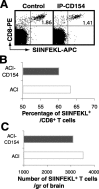
T-cell recruitment into the brain is critical in inflammatory and autoimmune diseases of the CNS. We use intracerebral antigen microinjection and tetramer technology to track antigen-specific CD8+ T-cells in the CNS and to clarify the contribution of antigen deposition or traumatic injury to the accumulation of T-cells in the brain. We demonstrate that, after intracerebral microinjection of ovalbumin, ovalbumin-specific CD8+ T-cells expand systemically and then migrate into the brain where they complete additional proliferation cycles. T-cells in the brain are activated and respond to in vitro secondary antigen challenge. CD8+ T-cells accumulate and persist in sites of antigen in the brain without replenishment from the periphery. Persistent survival of CD8+ T-cells at sites of cognate antigen is significantly reduced by blocking CD154 molecules. A small traumatic injury itself does not lead to recruitment of CD8+ T-cells into the brain but attracts activated antigen-specific CD8+ T-cells from cognate antigen injection sites. This process is presumably antigen independent and cannot be inhibited by blocking CD154 molecules. These data show that activated antigen-specific CD8+ T-cells accumulate in the CNS at both cognate antigen-containing and traumatic injury sites after intracerebral antigen delivery. The accumulation of activated antigen-specific T-cells at traumatic injury sites, in addition to antigen-containing areas, could amplify local inflammatory processes in the CNS. Combination therapies in neuroinflammatory diseases to block both of these processes should be considered.
Figures

Similar articles
-
In situ activation of antigen-specific CD8+ T cells in the presence of antigen in organotypic brain slices.J Immunol. 2008 Jun 15;180(12):8393-9. doi: 10.4049/jimmunol.180.12.8393. J Immunol. 2008. PMID: 18523307 Free PMC article.
-
Local Antigen Encounter Is Essential for Establishing Persistent CD8+ T-Cell Memory in the CNS.Front Immunol. 2019 Mar 4;10:351. doi: 10.3389/fimmu.2019.00351. eCollection 2019. Front Immunol. 2019. PMID: 30886617 Free PMC article.
-
In situ processing and distribution of intracerebrally injected OVA in the CNS.J Neuroimmunol. 2003 Aug;141(1-2):90-8. doi: 10.1016/s0165-5728(03)00249-2. J Neuroimmunol. 2003. PMID: 12965258
-
Disproportionate recruitment of CD8+ T cells into the central nervous system by professional antigen-presenting cells.Am J Pathol. 1999 Feb;154(2):481-94. doi: 10.1016/S0002-9440(10)65294-7. Am J Pathol. 1999. PMID: 10027406 Free PMC article.
-
An antigen-specific pathway for CD8 T cells across the blood-brain barrier.J Exp Med. 2007 Sep 3;204(9):2023-30. doi: 10.1084/jem.20070064. Epub 2007 Aug 6. J Exp Med. 2007. PMID: 17682068 Free PMC article.
Cited by
-
Immune cell trafficking from the brain maintains CNS immune tolerance.J Clin Invest. 2014 Mar;124(3):1228-41. doi: 10.1172/JCI71544. Epub 2014 Feb 24. J Clin Invest. 2014. PMID: 24569378 Free PMC article.
-
The level of B7 homologue 1 expression on brain DC is decisive for CD8 Treg cell recruitment into the CNS during EAE.Eur J Immunol. 2009 Jun;39(6):1536-43. doi: 10.1002/eji.200839165. Eur J Immunol. 2009. PMID: 19424967 Free PMC article.
-
Parasite stage-specific recognition of endogenous Toxoplasma gondii-derived CD8+ T cell epitopes.J Infect Dis. 2008 Dec 1;198(11):1625-33. doi: 10.1086/593019. J Infect Dis. 2008. PMID: 18922097 Free PMC article.
-
T cells in the central nervous system: messengers of destruction or purveyors of protection?Immunology. 2014 Mar;141(3):340-4. doi: 10.1111/imm.12187. Immunology. 2014. PMID: 24708415 Free PMC article. Review.
-
The role of macrophages in optic nerve regeneration.Neuroscience. 2009 Feb 6;158(3):1039-48. doi: 10.1016/j.neuroscience.2008.07.036. Epub 2008 Jul 25. Neuroscience. 2009. PMID: 18708126 Free PMC article. Review.
References
-
- Aktas O, Smorodchenko A, Brocke S, Infante-Duarte C, Topphoff US, Vogt J, Prozorovski T, Meier S, Osmanova V, Pohl E, Bechmann I, Nitsch R, Zipp F (2005) Neuronal damage in autoimmune neuroinflammation mediated by the death ligand TRAIL. Neuron 46: 421–432. - PubMed
-
- Arand M, Melzner H, Kinzl L, Bruckner UB, Gebhard F (2001) Early inflammatory mediator response following isolated traumatic brain injury and other major trauma in humans. Langenbecks Arch Surg 386: 241–248. - PubMed
-
- Azukizawa H, Kosaka H, Sano S, Heath WR, Takahashi I, Gao XH, Sumikawa Y, Okabe M, Yoshikawa K, Itami S (2003) Induction of T-cell-mediated skin disease specific for antigen transgenically expressed in keratinocytes. Eur J Immunol 33: 1879–1888. - PubMed
-
- Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schroder R, Deckert M, Schmidt S, Ravid R, Rajewsky K (2000) Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med 192: 393–404. - PMC - PubMed
-
- Booss J, Esiri MM, Tourtellotte WW, Mason DY (1983) Immunohistological analysis of T lymphocyte subsets in the central nervous system in chronic progressive multiple sclerosis. J Neurol Sci 62: 219–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
