Infection-dependent nuclear localization of US17, a member of the US12 family of human cytomegalovirus-encoded seven-transmembrane proteins
- PMID: 16414996
- PMCID: PMC1346967
- DOI: 10.1128/JVI.80.3.1191-1203.2006
Infection-dependent nuclear localization of US17, a member of the US12 family of human cytomegalovirus-encoded seven-transmembrane proteins
Abstract
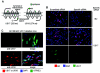
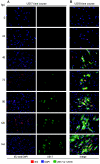
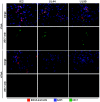
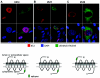
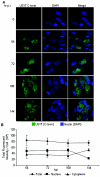
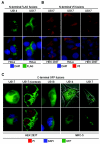
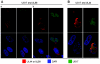
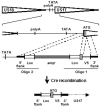
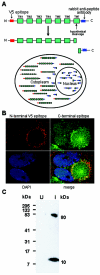
The human cytomegalovirus (HCMV) US12 gene family is a group of predicted seven-transmembrane, G-protein-coupled receptor-related proteins, about which little is known. Specific rabbit polyclonal antibodies detected US17 and US18 beginning 54 and 36 h after infection, respectively, with expression of both proteins dependent on viral DNA synthesis. While US14 and US18 are expressed exclusively in the cytoplasm, we unexpectedly found abundant expression of US17 in both the cytoplasm and nucleoplasm. N- and C-terminally tagged versions of US17 were readily detected in the cytoplasm of transfected mammalian cells, but not in nuclei, suggesting that nuclear localization involves other viral proteins or an infection-triggered cellular process. There was no specific colocalization between US17 and other nuclear expressed HCMV-encoded proteins (IE-2, DNA polymerase processivity factor, and pp28/UL99). To determine whether the observed nuclear localization might be the product of a process by which a soluble C-terminal segment of the full-length protein is expressed, we constructed a recombinant virus that incorporates a synthetic epitope at its N terminus, which in conjunction with the antipeptide antibody that targets its predicted cytoplasmic C-terminal segment, enables simultaneous independent detection of both termini. In cells infected with the recombinant, the US17 N and C termini had limited colocalization, with the N-terminal segment not detected in nuclei, supporting the segmentation hypothesis. Consistent with this, a fragment with an apparent molecular size of 10 kDa was detected by immunoblotting. We have identified the first viral example of a seven-transmembrane protein that is either segmented or expressed in nuclei. Further study will be required to learn the mechanism by which this occurs and the function of the nuclear localizing segment. This likely represents yet another mechanism by which a virus has hijacked or modified cellular regulatory pathways for its benefit.
Figures
Similar articles
-
A cluster of 3' coterminal transcripts from US12-US17 locus of human cytomegalovirus.Virus Genes. 2016 Jun;52(3):334-45. doi: 10.1007/s11262-016-1308-z. Epub 2016 Mar 1. Virus Genes. 2016. PMID: 26931512
-
Members of the HCMV US12 family of predicted heptaspanning membrane proteins have unique intracellular distributions, including association with the cytoplasmic virion assembly complex.Virology. 2007 May 10;361(2):263-73. doi: 10.1016/j.virol.2006.11.019. Epub 2006 Dec 22. Virology. 2007. PMID: 17188320
-
Primate cytomegalovirus US12 gene family: a distinct and diverse clade of seven-transmembrane proteins.Virology. 2006 Oct 25;354(2):286-98. doi: 10.1016/j.virol.2006.06.035. Epub 2006 Aug 10. Virology. 2006. PMID: 16904149
-
Humoral immune response to human cytomegalovirus proteins: a brief review.Comp Immunol Microbiol Infect Dis. 1991;14(2):97-105. doi: 10.1016/0147-9571(91)90124-v. Comp Immunol Microbiol Infect Dis. 1991. PMID: 1657515 Review.
-
The human cytomegalovirus chemokine receptor homolog encoded by US27.Virus Genes. 2017 Aug;53(4):516-521. doi: 10.1007/s11262-017-1462-y. Epub 2017 Apr 26. Virus Genes. 2017. PMID: 28447191 Review.
Cited by
-
Spatial relationships between markers for secretory and endosomal machinery in human cytomegalovirus-infected cells versus those in uninfected cells.J Virol. 2011 Jun;85(12):5864-79. doi: 10.1128/JVI.00155-11. Epub 2011 Apr 6. J Virol. 2011. PMID: 21471245 Free PMC article.
-
Infection of human cytomegalovirus in cultured human gingival tissue.Virol J. 2006 Oct 5;3:84. doi: 10.1186/1743-422X-3-84. Virol J. 2006. PMID: 17022821 Free PMC article.
-
Human Cytomegalovirus Replication and Infection-Induced Syncytia Formation in Labial, Foreskin, and Fetal Lung Fibroblasts.Viruses. 2021 Nov 24;13(12):2355. doi: 10.3390/v13122355. Viruses. 2021. PMID: 34960624 Free PMC article.
-
A cluster of 3' coterminal transcripts from US12-US17 locus of human cytomegalovirus.Virus Genes. 2016 Jun;52(3):334-45. doi: 10.1007/s11262-016-1308-z. Epub 2016 Mar 1. Virus Genes. 2016. PMID: 26931512
-
Two novel human cytomegalovirus NK cell evasion functions target MICA for lysosomal degradation.PLoS Pathog. 2014 May 1;10(5):e1004058. doi: 10.1371/journal.ppat.1004058. eCollection 2014 May. PLoS Pathog. 2014. PMID: 24787765 Free PMC article.
References
-
- Brown, M. S., J. Ye, R. B. Rawson, and J. L. Goldstein. 2000. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell 100:391-398. - PubMed
-
- Chambers, J., A. Angulo, D. Amaratunga, H. Guo, Y. Jiang, J. S. Wan, A. Bittner, K. Frueh, M. R. Jackson, P. A. Peterson, M. G. Erlander, and P. Ghazal. 1999. DNA microarrays of the complex human cytomegalovirus genome: profiling kinetic class with drug sensitivity of viral gene expression. J. Virol. 73:5757-5766. - PMC - PubMed
-
- Chee, M. S., A. T. Bankier, S. Beck, R. Bohni, C. M. Brown, R. Cerny, T. Horsnell, C. A. Hutchison, T. Kouzarides, J. A. Martignetti, E. Preddie, S. C. Satchwell, P. Tomlinson, K. M. Weston, and B. G. Barrell. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154:125-169. - PubMed
-
- Chen, R., Y. V. Mukhin, M. N. Garnovskaya, T. E. Thielen, Y. Iijima, C. Huang, J. R. Raymond, M. E. Ullian, and R. V. Paul. 2000. A functional angiotensin II receptor-GFP fusion protein: evidence for agonist-dependent nuclear translocation. Am. J. Physiol. Renal Physiol. 279:F440-F448. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

