SARS coronavirus nucleocapsid immunodominant T-cell epitope cluster is common to both exogenous recombinant and endogenous DNA-encoded immunogens
- PMID: 16387339
- PMCID: PMC7111852
- DOI: 10.1016/j.virol.2005.11.042
SARS coronavirus nucleocapsid immunodominant T-cell epitope cluster is common to both exogenous recombinant and endogenous DNA-encoded immunogens
Abstract
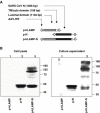
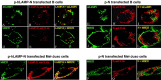
Correspondence between the T-cell epitope responses of vaccine immunogens and those of pathogen antigens is critical to vaccine efficacy. In the present study, we analyzed the spectrum of immune responses of mice to three different forms of the SARS coronavirus nucleocapsid (N): (1) exogenous recombinant protein (N-GST) with Freund's adjuvant; (2) DNA encoding unmodified N as an endogenous cytoplasmic protein (pN); and (3) DNA encoding N as a LAMP-1 chimera targeted to the lysosomal MHC II compartment (p-LAMP-N). Lysosomal trafficking of the LAMP/N chimera in transfected cells was documented by both confocal and immunoelectron microscopy. The responses of the immunized mice differed markedly. The strongest T-cell IFN-gamma and CTL responses were to the LAMP-N chimera followed by the pN immunogen. In contrast, N-GST elicited strong T cell IL-4 but minimal IFN-gamma responses and a much greater antibody response. Despite these differences, however, the immunodominant T-cell ELISpot responses to each of the three immunogens were elicited by the same N peptides, with the greatest responses being generated by a cluster of five overlapping peptides, N76-114, each of which contained nonameric H2d binding domains with high binding scores for both class I and, except for N76-93, class II alleles. These results demonstrate that processing and presentation of N, whether exogenously or endogenously derived, resulted in common immunodominant epitopes, supporting the usefulness of modified antigen delivery and trafficking forms and, in particular, LAMP chimeras as vaccine candidates. Nevertheless, the profiles of T-cell responses were distinctly different. The pronounced Th-2 and humoral response to N protein plus adjuvant are in contrast to the balanced IFN-gamma and IL-4 responses and strong memory CTL responses to the LAMP-N chimera.
Figures

Similar articles
-
Immunological characterizations of the nucleocapsid protein based SARS vaccine candidates.Vaccine. 2006 Apr 12;24(16):3100-8. doi: 10.1016/j.vaccine.2006.01.058. Epub 2006 Feb 8. Vaccine. 2006. PMID: 16494977 Free PMC article.
-
Immune responses to T-cell epitopes of SARS CoV-N protein are enhanced by N immunization with a chimera of lysosome-associated membrane protein.Gene Ther. 2009 Nov;16(11):1353-62. doi: 10.1038/gt.2009.92. Epub 2009 Sep 3. Gene Ther. 2009. PMID: 19727132 Free PMC article.
-
Immune responses against SARS-coronavirus nucleocapsid protein induced by DNA vaccine.Virology. 2005 Jan 5;331(1):128-35. doi: 10.1016/j.virol.2004.10.016. Virology. 2005. PMID: 15582659 Free PMC article.
-
Enhanced induction of SARS-CoV nucleocapsid protein-specific immune response using DNA vaccination followed by adenovirus boosting in BALB/c mice.Intervirology. 2006;49(5):307-18. doi: 10.1159/000094247. Epub 2006 Jun 29. Intervirology. 2006. PMID: 16809936 Free PMC article.
-
HLA-A*0201 T-cell epitopes in severe acute respiratory syndrome (SARS) coronavirus nucleocapsid and spike proteins.Biochem Biophys Res Commun. 2006 May 26;344(1):63-71. doi: 10.1016/j.bbrc.2006.03.152. Biochem Biophys Res Commun. 2006. PMID: 16630549 Free PMC article.
Cited by
-
Design and application of nanoparticles as vaccine adjuvants against human corona virus infection.J Inorg Biochem. 2021 Jun;219:111454. doi: 10.1016/j.jinorgbio.2021.111454. Epub 2021 Mar 29. J Inorg Biochem. 2021. PMID: 33878530 Free PMC article. Review.
-
Rabies DNA vaccine: no impact of MHC class I and class II targeting sequences on immune response and protection against lethal challenge.Vaccine. 2009 Mar 26;27(15):2128-37. doi: 10.1016/j.vaccine.2009.01.128. Epub 2009 Feb 6. Vaccine. 2009. PMID: 19356616 Free PMC article.
-
A decade after SARS: strategies for controlling emerging coronaviruses.Nat Rev Microbiol. 2013 Dec;11(12):836-48. doi: 10.1038/nrmicro3143. Epub 2013 Nov 11. Nat Rev Microbiol. 2013. PMID: 24217413 Free PMC article. Review.
-
Identification and characterization of dominant helper T-cell epitopes in the nucleocapsid protein of severe acute respiratory syndrome coronavirus.J Virol. 2007 Jun;81(11):6079-88. doi: 10.1128/JVI.02568-06. Epub 2007 Mar 28. J Virol. 2007. PMID: 17392374 Free PMC article.
-
Vaccines to prevent severe acute respiratory syndrome coronavirus-induced disease.Virus Res. 2008 Apr;133(1):45-62. doi: 10.1016/j.virusres.2007.01.021. Epub 2007 Apr 9. Virus Res. 2008. PMID: 17416434 Free PMC article.
References
-
- Ackerman A.L., Cresswell P. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat. Immunol. 2004;5(7):678–684. - PubMed
-
- Anwar A., Chandrasekaran A., Lee Ng M., Marques E., August J.T. West Nile premembrane-envelope genetic vaccine encoded as a chimera containing the transmembrane and cytoplasmic domains of a lysosome associated membrane protein: increased cellular concentration of the transgene product, targeting to the MHC II compartment and enhanced neutralizing antibody response. J. Virol. 2005;332(1):66–77. - PubMed
-
- Barros de Arruda L., Chikhlikar P.R., August J.T., Marques E.T. DNA vaccine encoding human immunodeficiency virus-1 Gag, targeted to the major histocompatibility complex II compartment by lysosomal-associated membrane protein, elicits enhanced long-term memory response. Immunol. 2004;112(1):126–133. - PMC - PubMed
-
- Benacerraf B. A hypothesis to relate the specificity of T lymphocytes and the activity of I region-specific Ir genes in macrophages and B lymphocytes. J. Immunol. 1978;120(6):1809–1812. - PubMed
-
- Berzofsky J.A., Pendleton C.D., Clerici M., Ahlers J., Lucey D.R., Putney S.D., Shearer G.M. Construction of peptides encompassing multideterminant clusters of human immunodeficiency virus envelope to induce in vitro T cell responses in mice and humans of multiple MHC types. J. Clin. Invest. 1991;88(3):876–884. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

