LAT-mediated signaling in CD4+CD25+ regulatory T cell development
- PMID: 16380508
- PMCID: PMC2118069
- DOI: 10.1084/jem.20050903
LAT-mediated signaling in CD4+CD25+ regulatory T cell development
Abstract
Engagement of the T cell receptor for antigen (TCR) induces formation of signaling complexes mediated through the transmembrane adaptor protein, the linker for activation of T cells (LAT). LAT plays an important role in T cell development, activation, and homeostasis. A knock-in mutation at Tyr136, which is the phospholipase C (PLC)-gamma1-binding site in LAT, leads to a severe autoimmune disease in mice. In this study, we show that CD4+CD25+ T reg cells that expressed Foxp3 transcription factor were nearly absent in both thymus and peripheral lymphoid organs of LAT(Y136F) mice. This defect was not a result of the autoimmune environment as LAT(Y136F) T reg cells also failed to develop in healthy LAT-/- mice that received mixed wild-type and LAT(Y136F) bone marrow cells. Moreover, adoptive transfer of normal CD4+CD25+ T reg cells protected neonatal LAT(Y136F) mice from developing this disease. These T reg cells effectively controlled expansion of CD4+ T cells in LAT(Y136F) mice likely via granzymes and/or TGF-beta-mediated suppression. Furthermore, ectopic expression of Foxp3 conferred a suppressive function in LAT(Y136F) T cells. Our data indicate that the LAT-PLC-gamma1 interaction plays a critical role in Foxp3 expression and the development of CD4+CD25+ T reg cells.
Figures



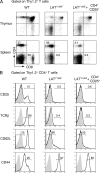
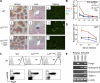
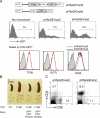
Similar articles
-
The Importance of IL-6 in the Development of LAT-Mediated Autoimmunity.J Immunol. 2015 Jul 15;195(2):695-705. doi: 10.4049/jimmunol.1403187. Epub 2015 Jun 1. J Immunol. 2015. PMID: 26034173 Free PMC article.
-
Th2 lymphoproliferative disorder of LatY136F mutant mice unfolds independently of TCR-MHC engagement and is insensitive to the action of Foxp3+ regulatory T cells.J Immunol. 2008 Feb 1;180(3):1565-75. doi: 10.4049/jimmunol.180.3.1565. J Immunol. 2008. PMID: 18209052
-
Loss of the LAT adaptor converts antigen-responsive T cells into pathogenic effectors that function independently of the T cell receptor.Immunity. 2009 Aug 21;31(2):197-208. doi: 10.1016/j.immuni.2009.05.013. Epub 2009 Aug 13. Immunity. 2009. PMID: 19682930
-
Role of the LAT adaptor in T-cell development and Th2 differentiation.Adv Immunol. 2005;87:1-25. doi: 10.1016/S0065-2776(05)87001-4. Adv Immunol. 2005. PMID: 16102570 Review.
-
Lymphoproliferative disorders involving T helper effector cells with defective LAT signalosomes.Semin Immunopathol. 2010 Jun;32(2):117-25. doi: 10.1007/s00281-009-0195-y. Epub 2010 Jan 28. Semin Immunopathol. 2010. PMID: 20107804 Review.
Cited by
-
Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets.Immunol Rev. 2013 Mar;252(1):12-23. doi: 10.1111/imr.12032. Immunol Rev. 2013. PMID: 23405892 Free PMC article. Review.
-
Immune regulation and control of regulatory T cells by OX40 and 4-1BB.Cytokine Growth Factor Rev. 2008 Jun-Aug;19(3-4):253-62. doi: 10.1016/j.cytogfr.2008.04.003. Epub 2008 May 27. Cytokine Growth Factor Rev. 2008. PMID: 18508403 Free PMC article. Review.
-
Integrative biology of T cell activation.Nat Immunol. 2014 Sep;15(9):790-7. doi: 10.1038/ni.2959. Nat Immunol. 2014. PMID: 25137453 Review.
-
The Importance of IL-6 in the Development of LAT-Mediated Autoimmunity.J Immunol. 2015 Jul 15;195(2):695-705. doi: 10.4049/jimmunol.1403187. Epub 2015 Jun 1. J Immunol. 2015. PMID: 26034173 Free PMC article.
-
Antigen exposure shapes the ratio between antigen-specific Tregs and conventional T cells in human peripheral blood.Proc Natl Acad Sci U S A. 2016 Oct 11;113(41):E6192-E6198. doi: 10.1073/pnas.1611723113. Epub 2016 Sep 28. Proc Natl Acad Sci U S A. 2016. PMID: 27681619 Free PMC article.
References
-
- Sakaguchi, S. 2000. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 101:455–458. - PubMed
-
- Shevach, E.M., R.S. McHugh, C.A. Piccirillo, and A.M. Thornton. 2001. Control of T-cell activation by CD4+CD25+ suppressor T cells. Immunol. Rev. 182:58–67. - PubMed
-
- Bluestone, J.A., and A.K. Abbas. 2003. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 3:253–257. - PubMed
-
- Papiernik, M., M. de Moraes, C. Pontoux, F. Vasseur, and C. Penit. 1998. Regulatory CD4 T cells: expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int. Immunol. 10:371–378. - PubMed
-
- Itoh, M., T. Takahashi, N. Sakaguchi, Y. Kuniyasu, J. Shimizu, F. Otsuka, and S. Sakaguchi. 1999. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162:5317–5326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

