Adenovirus E1B 55-kilodalton protein is required for both regulation of mRNA export and efficient entry into the late phase of infection in normal human fibroblasts
- PMID: 16378998
- PMCID: PMC1346875
- DOI: 10.1128/JVI.80.2.964-974.2006
Adenovirus E1B 55-kilodalton protein is required for both regulation of mRNA export and efficient entry into the late phase of infection in normal human fibroblasts
Abstract
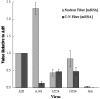
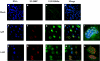
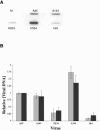
The human adenovirus type 5 (Ad5) E1B 55-kDa protein is required for selective nuclear export of viral late mRNAs from the nucleus and concomitant inhibition of export of cellular mRNAs in HeLa cells and some other human cell lines, but its contributions(s) to replication in normal human cells is not well understood. We have therefore examined the phenotypes exhibited by viruses carrying mutations in the E1B 55-kDa protein coding sequence in normal human fibroblast (HFFs). Ad5 replicated significantly more slowly in HFFs than it does in tumor cells, a difference that is the result of delayed entry into the late phase of infection. The A143 mutation, which specifically impaired export of viral late mRNAs from the nucleus in infected HeLa cells (R. A. Gonzalez and S. J. Flint, J. Virol. 76:4507-4519, 2002), induced a more severe defect in viral mRNA export in HFFs. This observation indicates that the E1B 55-kDa protein regulates mRNA export during the late phase of infection of normal human cells. Other mutants exhibited phenotypes not observed in HeLa cells. In HFFs infected by the null mutant Hr6, synthesis of viral late mRNAs and proteins was severely impaired. Such defects in late gene expression were the result of inefficient progression into the late phase of infection, for viral DNA synthesis was 10-fold less efficient in Hr6-infected HFFs than in cells infected by Ad5. Similar, but less severe, defects in viral DNA synthesis were induced by the insertion mutation H224, which has been reported to inhibit binding of the E1B 55-kDa protein to p53 (C. C. Kao, P. R. Yew, and A. J. Berk, Virology 179:806-814, 1990).
Figures
Similar articles
-
An early function of the adenoviral E1B 55 kDa protein is required for the nuclear relocalization of the cellular p53 protein in adenovirus-infected normal human cells.Virology. 2008 Sep 1;378(2):339-46. doi: 10.1016/j.virol.2008.06.016. Epub 2008 Jul 15. Virology. 2008. PMID: 18632130
-
Effects of mutations in the adenoviral E1B 55-kilodalton protein coding sequence on viral late mRNA metabolism.J Virol. 2002 May;76(9):4507-19. doi: 10.1128/jvi.76.9.4507-4519.2002. J Virol. 2002. PMID: 11932416 Free PMC article.
-
Export of adenoviral late mRNA from the nucleus requires the Nxf1/Tap export receptor.J Virol. 2011 Feb;85(4):1429-38. doi: 10.1128/JVI.02108-10. Epub 2010 Dec 1. J Virol. 2011. PMID: 21123381 Free PMC article.
-
Regulation of mRNA production by the adenoviral E1B 55-kDa and E4 Orf6 proteins.Curr Top Microbiol Immunol. 2003;272:287-330. doi: 10.1007/978-3-662-05597-7_10. Curr Top Microbiol Immunol. 2003. PMID: 12747554 Review.
-
The biology of the adenovirus E1B 55K protein.FEBS Lett. 2019 Dec;593(24):3504-3517. doi: 10.1002/1873-3468.13694. Epub 2019 Dec 8. FEBS Lett. 2019. PMID: 31769868 Review.
Cited by
-
Reduced infectivity of adenovirus type 5 particles and degradation of entering viral genomes associated with incomplete processing of the preterminal protein.J Virol. 2012 Dec;86(24):13554-65. doi: 10.1128/JVI.02337-12. Epub 2012 Oct 3. J Virol. 2012. PMID: 23035217 Free PMC article.
-
RUNX1 permits E4orf6-directed nuclear localization of the adenovirus E1B-55K protein and associates with centers of viral DNA and RNA synthesis.J Virol. 2008 Jul;82(13):6395-408. doi: 10.1128/JVI.00043-08. Epub 2008 Apr 16. J Virol. 2008. PMID: 18417565 Free PMC article.
-
Tumor associated stromal cells play a critical role on the outcome of the oncolytic efficacy of conditionally replicative adenoviruses.PLoS One. 2009;4(4):e5119. doi: 10.1371/journal.pone.0005119. Epub 2008 Apr 8. PLoS One. 2009. PMID: 19337591 Free PMC article.
-
Normal human cell proteins that interact with the adenovirus type 5 E1B 55kDa protein.Virology. 2017 Apr;504:12-24. doi: 10.1016/j.virol.2017.01.013. Epub 2017 Jan 27. Virology. 2017. PMID: 28135605 Free PMC article.
-
The p53 protein does not facilitate adenovirus type 5 replication in normal human cells.J Virol. 2013 May;87(10):6044-6. doi: 10.1128/JVI.00129-13. Epub 2013 Mar 13. J Virol. 2013. PMID: 23487462 Free PMC article.
References
-
- Reference deleted.
-
- Aiello, L., R. Guilfoyle, K. Huebner, and R. Weinmann. 1979. Adenovirus 5 DNA sequences present and RNA sequences transcribed in transformed human embryo kidney cells (HEK-Ad-5 or 293). Virology 94:460-469. - PubMed
-
- Aspegren, A., C. Rabino, and E. Bridge. 1998. Organization of splicing factors in adenovirus-infected cells reflects changes in gene expression during the early to late phase transition. Exp. Cell Res. 245:203-213. - PubMed
-
- Barker, D. D., and A. J. Berk. 1987. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology 156:107-121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

