Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant
- PMID: 16378985
- PMCID: PMC1346878
- DOI: 10.1128/JVI.80.2.835-844.2006
Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant
Erratum in
- J Virol. 2006 Mar;80(5):2585
Abstract
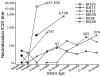
Maternal passive immunity typically plays a critical role in protecting infants from new infections; however, the specific contribution of neutralizing antibodies in limiting mother-to-child transmission of human immunodeficiency virus type 1 is unclear. By examining cloned envelope variants from 12 transmission pairs, we found that vertically transmitted variants were more resistant to neutralization by maternal plasma than were maternal viral variants near the time of transmission. The vertically transmitted envelope variants were poorly neutralized by monoclonal antibodies b12 [corrected] 2G12, 2F5, and 4E10 individually or in combination. Despite the fact that the infant viruses were among the most neutralization resistant in the mother, they had relatively few glycosylation sites. Moreover, the transmitted variants elicited de novo neutralizing antibodies in the infants, indicating that they were not inherently difficult to neutralize. The neutralization resistance of vertically transmitted viruses is in contrast to the relative neutralization sensitivity of viruses sexually transmitted within discordant couples, suggesting that the antigenic properties of viruses that are favored for transmission may differ depending upon mode of transmission.
Figures



Similar articles
-
Susceptibility of recently transmitted subtype B human immunodeficiency virus type 1 variants to broadly neutralizing antibodies.J Virol. 2007 Aug;81(16):8533-42. doi: 10.1128/JVI.02816-06. Epub 2007 May 23. J Virol. 2007. PMID: 17522228 Free PMC article.
-
Neutralizing antibody escape during HIV-1 mother-to-child transmission involves conformational masking of distal epitopes in envelope.J Virol. 2012 Sep;86(18):9566-82. doi: 10.1128/JVI.00953-12. Epub 2012 Jun 27. J Virol. 2012. PMID: 22740394 Free PMC article.
-
Short communication: HIV type 1 subtype C variants transmitted through the bottleneck of breastfeeding are sensitive to new generation broadly neutralizing antibodies directed against quaternary and CD4-binding site epitopes.AIDS Res Hum Retroviruses. 2013 Mar;29(3):511-5. doi: 10.1089/AID.2012.0197. Epub 2013 Jan 18. AIDS Res Hum Retroviruses. 2013. PMID: 23075434 Free PMC article.
-
Transmission of HIV-1 in the face of neutralizing antibodies.Curr HIV Res. 2007 Nov;5(6):578-87. doi: 10.2174/157016207782418461. Curr HIV Res. 2007. PMID: 18045114 Review.
-
Antibodies for prevention of mother-to-child transmission of HIV-1.Curr Opin HIV AIDS. 2015 May;10(3):177-82. doi: 10.1097/COH.0000000000000150. Curr Opin HIV AIDS. 2015. PMID: 25700205 Free PMC article. Review.
Cited by
-
HIV-1 autologous antibody neutralization associates with mother to child transmission.PLoS One. 2013 Jul 17;8(7):e69274. doi: 10.1371/journal.pone.0069274. Print 2013. PLoS One. 2013. PMID: 23874931 Free PMC article.
-
Evaluation of repRNA vaccine for induction and in utero transfer of maternal antibodies in a pregnant rabbit model.Mol Ther. 2023 Apr 5;31(4):1046-1058. doi: 10.1016/j.ymthe.2023.02.022. Epub 2023 Mar 24. Mol Ther. 2023. PMID: 36965482 Free PMC article.
-
Neutralizing and other antiviral antibodies in HIV-1 infection and vaccination.Curr Opin HIV AIDS. 2007 May;2(3):169-76. doi: 10.1097/COH.0b013e3280ef691e. Curr Opin HIV AIDS. 2007. PMID: 19372883 Free PMC article.
-
The HIV-1 Envelope Glycoprotein C3/V4 Region Defines a Prevalent Neutralization Epitope following Immunization.Cell Rep. 2019 Apr 9;27(2):586-598.e6. doi: 10.1016/j.celrep.2019.03.039. Cell Rep. 2019. PMID: 30970260 Free PMC article.
-
An Asymmetric Opening of HIV-1 Envelope Mediates Antibody-Dependent Cellular Cytotoxicity.Cell Host Microbe. 2019 Apr 10;25(4):578-587.e5. doi: 10.1016/j.chom.2019.03.002. Cell Host Microbe. 2019. PMID: 30974085 Free PMC article.
References
-
- Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. - PubMed
-
- Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78:13232-13252. - PMC - PubMed
-
- Bongertz, V., C. I. Costa, V. G. Veloso, B. Grinsztejn, E. C. Filho, G. Calvet, and J. H. Pilotto. 2002. Neutralization titres and vertical HIV-1 transmission. Scand. J. Immunol. 56:642-644. - PubMed
-
- Buchacher, A., R. Predl, K. Strutzenberger, W. Steinfellner, A. Trkola, M. Purtscher, G. Gruber, C. Tauer, F. Steindl, A. Jungbauer, and H. Katinger. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retrovir. 10:359-369. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

