Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion
- PMID: 16378974
- PMCID: PMC1346879
- DOI: 10.1128/JVI.80.2.710-722.2006
Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion
Abstract
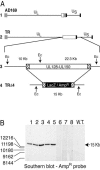
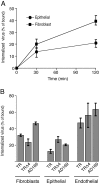
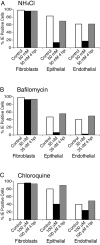
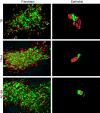
Human cytomegalovirus (HCMV) replication in epithelial and endothelial cells appears to be important in virus spread, disease, and persistence. It has been difficult to study infection of these cell types because HCMV laboratory strains (e.g., AD169 and Towne) have lost their ability to infect cultured epithelial and endothelial cells during extensive propagation in fibroblasts. Clinical strains of HCMV (e.g., TR and FIX) possess a cluster of genes (UL128 to UL150) that are largely mutated in laboratory strains, and recent studies have indicated that these genes facilitate replication in epithelial and endothelial cells. The mechanisms by which these genes promote infection of these two cell types are unclear. We derived an HCMV UL128-to-UL150 deletion mutant from strain TR, TRdelta4, and studied early events in HCMV infection of epithelial and endothelial cells, and the role of genes UL128 to UL150. Analysis of wild-type TR indicated that HCMV enters epithelial and endothelial cells by endocytosis followed by low-pH-dependent fusion, which is different from the pH-independent fusion with the plasma membrane observed with human fibroblasts. TRdelta4 displayed a number of defects in early infection processes. Adsorption and entry of TRdelta4 on epithelial cells were poor compared with those of TR, but these defects could be overcome with higher doses of virus and the use of polyethylene glycol (PEG) to promote fusion between virion and cellular membranes. High multiplicity and PEG treatment did not promote infection of endothelial cells by TRdelta4, yet virus particles were internalized. Together, these data indicate that genes UL128 to UL150 are required for HCMV adsorption and penetration of epithelial cells and to promote some early stage of virus replication, subsequent to virus entry, in endothelial cells.
Figures


Similar articles
-
A human cytomegalovirus gO-null mutant fails to incorporate gH/gL into the virion envelope and is unable to enter fibroblasts and epithelial and endothelial cells.J Virol. 2010 Mar;84(5):2585-96. doi: 10.1128/JVI.02249-09. Epub 2009 Dec 23. J Virol. 2010. PMID: 20032184 Free PMC article.
-
PDGF receptor-α does not promote HCMV entry into epithelial and endothelial cells but increased quantities stimulate entry by an abnormal pathway.PLoS Pathog. 2012 Sep;8(9):e1002905. doi: 10.1371/journal.ppat.1002905. Epub 2012 Sep 13. PLoS Pathog. 2012. PMID: 23028311 Free PMC article.
-
Loss of the Human Cytomegalovirus US16 Protein Abrogates Virus Entry into Endothelial and Epithelial Cells by Reducing the Virion Content of the Pentamer.J Virol. 2017 May 12;91(11):e00205-17. doi: 10.1128/JVI.00205-17. Print 2017 Jun 1. J Virol. 2017. PMID: 28331097 Free PMC article.
-
Human cytomegalovirus tropism for endothelial/epithelial cells: scientific background and clinical implications.Rev Med Virol. 2010 May;20(3):136-55. doi: 10.1002/rmv.645. Rev Med Virol. 2010. PMID: 20084641 Review.
-
Endothelial cells in human cytomegalovirus infection: one host cell out of many or a crucial target for virus spread?Thromb Haemost. 2009 Dec;102(6):1057-63. doi: 10.1160/TH09-04-0213. Thromb Haemost. 2009. PMID: 19967135 Review.
Cited by
-
Development of a Vaccine against Human Cytomegalovirus: Advances, Barriers, and Implications for the Clinical Practice.Vaccines (Basel). 2021 May 25;9(6):551. doi: 10.3390/vaccines9060551. Vaccines (Basel). 2021. PMID: 34070277 Free PMC article. Review.
-
Comparative analysis of gO isoforms reveals that strains of human cytomegalovirus differ in the ratio of gH/gL/gO and gH/gL/UL128-131 in the virion envelope.J Virol. 2013 Sep;87(17):9680-90. doi: 10.1128/JVI.01167-13. Epub 2013 Jun 26. J Virol. 2013. PMID: 23804643 Free PMC article.
-
Virus-Induced Cell Fusion and Syncytia Formation.Results Probl Cell Differ. 2024;71:283-318. doi: 10.1007/978-3-031-37936-9_14. Results Probl Cell Differ. 2024. PMID: 37996683
-
Human cytomegalovirus glycoprotein B variants affect viral entry, cell fusion, and genome stability.Proc Natl Acad Sci U S A. 2019 Sep 3;116(36):18021-18030. doi: 10.1073/pnas.1907447116. Epub 2019 Aug 19. Proc Natl Acad Sci U S A. 2019. PMID: 31427511 Free PMC article.
-
Conformational Changes in Herpes Simplex Virus Glycoprotein C.J Virol. 2022 Aug 24;96(16):e0016322. doi: 10.1128/jvi.00163-22. Epub 2022 Aug 1. J Virol. 2022. PMID: 35913218 Free PMC article.
References
-
- Bissinger, A. L., C. Sinzger, E. Kaiserling, and G. Jahn. 2002. Human cytomegalovirus as a direct pathogen: correlation of multiorgan involvement and cell distribution with clinical and pathological findings in a case of congenital inclusion disease. J. Med. Virol. 67:200-206. - PubMed
-
- Bodaghi, B., M. E. P. Slobbe-Vandrunen, A. Topilko, E. Perret, R. C. R. M. Vossen, M. C. E. Van Dam-Mieras, D. Zipeto, J.-L. Virelizier, P. Lehoang, C. A. Bruggeman, and S. Michelson. 1999. Entry of human cytomegalovirus into retinal pigment epithelial and endothelial cells by endocytosis. Investig. Ophthalmol. Vis. Sci. 40:2598-2607. - PubMed
-
- Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594-599. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

