Anti-Abeta42- and anti-Abeta40-specific mAbs attenuate amyloid deposition in an Alzheimer disease mouse model
- PMID: 16341263
- PMCID: PMC1307561
- DOI: 10.1172/JCI25410
Anti-Abeta42- and anti-Abeta40-specific mAbs attenuate amyloid deposition in an Alzheimer disease mouse model
Abstract
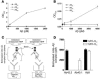
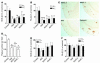
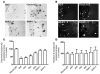
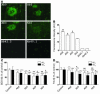
Accumulation and aggregation of amyloid beta peptide 1-42 (Abeta42) in the brain has been hypothesized as triggering a pathological cascade that causes Alzheimer disease (AD). To determine whether selective targeting of Abeta42 versus Abeta40 or total Abeta is an effective way to prevent or treat AD, we compared the effects of passive immunization with an anti-Abeta42 mAb, an anti-Abeta40 mAb, and multiple Abeta(1-16) mAbs. We established in vivo binding selectivity of the anti-Abeta42 and anti-Abeta40 mAbs using novel TgBRI-Abeta mice. We then conducted a prevention study in which the anti-Abeta mAbs were administered to young Tg2576 mice, which have no significant Abeta deposition, and therapeutic studies in which mAbs were administered to Tg2576 or CRND8 mice with modest levels of preexisting Abeta deposits. Anti-Abeta42, anti-Abeta40, and anti-Abeta(1-16) mAbs attenuated plaque deposition in the prevention study. In contrast, anti-Abeta42 and anti-Abeta40 mAbs were less effective in attenuating Abeta deposition in the therapeutic studies and were not effective in clearing diffuse plaques following direct injection into the cortex. These data suggest that selective targeting of Abeta42 or Abeta40 may be an effective strategy to prevent amyloid deposition, but may have limited benefit in a therapeutic setting.
Figures

Similar articles
-
Reduced effectiveness of Abeta1-42 immunization in APP transgenic mice with significant amyloid deposition.Neurobiol Aging. 2001 Sep-Oct;22(5):721-7. doi: 10.1016/s0197-4580(01)00245-7. Neurobiol Aging. 2001. PMID: 11705631
-
Passive immunization of the Abeta42(43) C-terminal-specific antibody BC05 in a mouse model of Alzheimer's disease.Neurodegener Dis. 2005;2(1):36-43. doi: 10.1159/000086429. Neurodegener Dis. 2005. PMID: 16909001
-
Lack of P-glycoprotein Results in Impairment of Removal of Beta-Amyloid and Increased Intraparenchymal Cerebral Amyloid Angiopathy after Active Immunization in a Transgenic Mouse Model of Alzheimer's Disease.Curr Alzheimer Res. 2017;14(6):656-667. doi: 10.2174/1567205013666161201201227. Curr Alzheimer Res. 2017. PMID: 27915995
-
Intracerebroventricular passive immunization in transgenic mouse models of Alzheimer's disease.Expert Rev Vaccines. 2004 Dec;3(6):717-25. doi: 10.1586/14760584.3.6.717. Expert Rev Vaccines. 2004. PMID: 15606357 Review.
-
Immunotherapy of Alzheimer's disease (AD): from murine models to anti-amyloid beta (Abeta) human monoclonal antibodies.Autoimmun Rev. 2006 Jan;5(1):33-9. doi: 10.1016/j.autrev.2005.06.007. Epub 2005 Aug 1. Autoimmun Rev. 2006. PMID: 16338209 Review.
Cited by
-
The stress response neuropeptide CRF increases amyloid-β production by regulating γ-secretase activity.EMBO J. 2015 Jun 12;34(12):1674-86. doi: 10.15252/embj.201488795. Epub 2015 May 11. EMBO J. 2015. PMID: 25964433 Free PMC article.
-
Molecular basis for passive immunotherapy of Alzheimer's disease.Proc Natl Acad Sci U S A. 2007 Oct 2;104(40):15659-64. doi: 10.1073/pnas.0705888104. Epub 2007 Sep 25. Proc Natl Acad Sci U S A. 2007. PMID: 17895381 Free PMC article.
-
Biomarkers for Alzheimer's disease: academic, industry and regulatory perspectives.Nat Rev Drug Discov. 2010 Jul;9(7):560-74. doi: 10.1038/nrd3115. Nat Rev Drug Discov. 2010. PMID: 20592748 Review.
-
Neuroprotective natural antibodies to assemblies of amyloidogenic peptides decrease with normal aging and advancing Alzheimer's disease.Proc Natl Acad Sci U S A. 2009 Jul 21;106(29):12145-50. doi: 10.1073/pnas.0904866106. Epub 2009 Jul 6. Proc Natl Acad Sci U S A. 2009. PMID: 19581601 Free PMC article.
-
Complex relationships between substrate sequence and sensitivity to alterations in γ-secretase processivity induced by γ-secretase modulators.Biochemistry. 2014 Apr 1;53(12):1947-57. doi: 10.1021/bi401521t. Epub 2014 Mar 20. Biochemistry. 2014. PMID: 24620716 Free PMC article.
References
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Iwatsubo T, et al. Visualization of A beta 42(43) and A beta 40 in senile plaques with and-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13:45–53. - PubMed
-
- Gravina SA, et al. Amyloid beta protein (A beta) in Alzheimer’s disease brain. Biochemical and immunocytochemical analysis with antibodies specific for forms ending at A beta 40 or A beta 42(43) J. Biol. Chem. 1995;270:7013–7016. - PubMed
-
- Golde TE, Eckman CB, Younkin SG. Biochemical detection of Abeta isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer’s disease. . Biochim. Biophys. Acta. 2000; 1502:172–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

