Novel Abeta peptide immunogens modulate plaque pathology and inflammation in a murine model of Alzheimer's disease
- PMID: 16332263
- PMCID: PMC1326209
- DOI: 10.1186/1742-2094-2-28
Novel Abeta peptide immunogens modulate plaque pathology and inflammation in a murine model of Alzheimer's disease
Abstract
Background: Alzheimer's disease, a common dementia of the elder, is characterized by accumulation of protein amyloid deposits in the brain. Immunization to prevent this accumulation has been proposed as a therapeutic possibility, although adverse inflammatory reactions in human trials indicate the need for novel vaccination strategies.
Method: Here vaccination with novel amyloid peptide immunogens was assessed in a transgenic mouse model displaying age-related accumulation of fibrillar plaques.
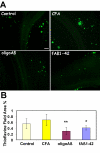
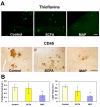
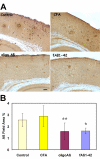
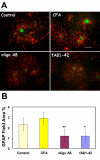
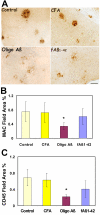
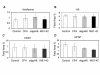
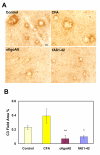
Results: Immunization with any conformation of the amyloid peptide initiated at 12 months of age (at which time fibrillar amyloid has just begun to accumulate) showed significant decrease in total and fibrillar amyloid deposits and in glial reactivity relative to control transgenic animals. In contrast, there was no significant decrease in amyloid deposition or glial activation in mice in which vaccination was initiated at 16 months of age, despite the presence of similar levels anti-Abeta antibodies in young and old animals vaccinated with a given immunogen. Interestingly, immunization with an oligomeric conformation of Abeta was equally as effective as other amyloid peptides at reducing plaque accumulation. However, the antibodies generated by immunization with the oligomeric conformation of Abeta have more limited epitope reactivity than those generated by fAbeta, and the microglial response was significantly less robust.
Conclusion: These results suggest that a more specific immunogen such as oligomeric Abeta can be designed that achieves the goal of depleting amyloid while reducing potential detrimental inflammatory reactions. In addition, the data show that active immunization of older Tg2576 mice with any amyloid conformation is not as efficient at reducing amyloid accumulation and related pathology as immunization of younger mice, and that serum anti-amyloid antibody levels are not quantitatively related to reduced amyloid-associated pathology.
Figures

Similar articles
-
Alzheimer's disease amyloid-β pathology in the lens of the eye.Exp Eye Res. 2022 Aug;221:108974. doi: 10.1016/j.exer.2022.108974. Epub 2022 Feb 21. Exp Eye Res. 2022. PMID: 35202705 Free PMC article.
-
Alzheimer's disease peptide epitope vaccine reduces insoluble but not soluble/oligomeric Abeta species in amyloid precursor protein transgenic mice.J Neurosci. 2007 Nov 14;27(46):12721-31. doi: 10.1523/JNEUROSCI.3201-07.2007. J Neurosci. 2007. PMID: 18003852 Free PMC article.
-
Nonhuman amyloid oligomer epitope reduces Alzheimer's-like neuropathology in 3xTg-AD transgenic mice.Mol Neurobiol. 2013 Dec;48(3):931-40. doi: 10.1007/s12035-013-8478-7. Epub 2013 Jun 15. Mol Neurobiol. 2013. PMID: 23771815
-
Developing novel immunogens for a safe and effective Alzheimer's disease vaccine.Prog Brain Res. 2009;175:83-93. doi: 10.1016/S0079-6123(09)17506-4. Prog Brain Res. 2009. PMID: 19660650 Free PMC article. Review.
-
Novel Abeta immunogens: is shorter better?Curr Alzheimer Res. 2007 Sep;4(4):427-36. doi: 10.2174/156720507781788800. Curr Alzheimer Res. 2007. PMID: 17908047 Review.
Cited by
-
Vaccine Development to Treat Alzheimer's Disease Neuropathology in APP/PS1 Transgenic Mice.Int J Alzheimers Dis. 2012;2012:376138. doi: 10.1155/2012/376138. Epub 2012 Sep 16. Int J Alzheimers Dis. 2012. PMID: 23024882 Free PMC article.
-
The MultiTEP platform-based Alzheimer's disease epitope vaccine activates a broad repertoire of T helper cells in nonhuman primates.Alzheimers Dement. 2014 May;10(3):271-83. doi: 10.1016/j.jalz.2013.12.003. Epub 2014 Feb 20. Alzheimers Dement. 2014. PMID: 24560029 Free PMC article.
-
Boosting with intranasal dendrimeric Abeta1-15 but not Abeta1-15 peptide leads to an effective immune response following a single injection of Abeta1-40/42 in APP-tg mice.J Neuroinflammation. 2006 Jun 5;3:14. doi: 10.1186/1742-2094-3-14. J Neuroinflammation. 2006. PMID: 16753065 Free PMC article.
-
Impact of amyloid imaging on drug development in Alzheimer's disease.Nucl Med Biol. 2007 Oct;34(7):809-22. doi: 10.1016/j.nucmedbio.2007.06.015. Epub 2007 Sep 4. Nucl Med Biol. 2007. PMID: 17921032 Free PMC article. Review.
-
Vaccination with a non-human random sequence amyloid oligomer mimic results in improved cognitive function and reduced plaque deposition and micro hemorrhage in Tg2576 mice.Mol Neurodegener. 2012 Aug 6;7:37. doi: 10.1186/1750-1326-7-37. Mol Neurodegener. 2012. PMID: 22866920 Free PMC article.
References
-
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang JP, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao ZM, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D. Immunization with amyloid-b attenuates Alzheimer disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. - DOI - PubMed
-
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. - DOI - PubMed

