Overexpression of the epidermal growth factor receptor confers migratory properties to nonmigratory postnatal neural progenitors
- PMID: 16319309
- PMCID: PMC6725641
- DOI: 10.1523/JNEUROSCI.2981-05.2005
Overexpression of the epidermal growth factor receptor confers migratory properties to nonmigratory postnatal neural progenitors
Abstract
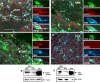
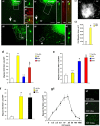
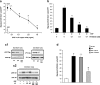
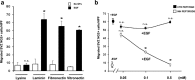
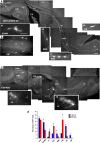
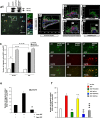
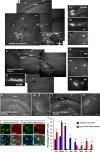
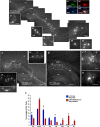
Approaches to successful cell transplantation therapies for the injured brain involve selecting the appropriate neural progenitor type and optimizing the efficiency of the cell engraftment. Here we show that epidermal growth factor receptor (EGFR) expression enhances postnatal neural progenitor migration in vitro and in vivo. Migratory NG2-expressing (NG2+) progenitor cells of the postnatal subventricular zone (SVZ) express higher EGFR levels than nonmigratory, cortical NG2+ cells. The higher endogenous EGFR expression in SVZ NG2+ cells is causally related with their migratory potential in vitro as well as in vivo after cell engraftment. EGFR overexpression in cortical NG2+ cells by transient transfection converted these cells to a migratory phenotype in vitro and in vivo. Finally, cortical NG2+ cells purified from a transgenic mouse in which the EGFR is overexpressed under the CNP promoter exhibited enhanced migratory capability. These findings reveal a new role for EGFR in the postnatal brain and open new avenues to optimize cell engraftment for brain repair.
Figures
Similar articles
-
Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone.J Neurosci. 2004 Nov 17;24(46):10530-41. doi: 10.1523/JNEUROSCI.3572-04.2004. J Neurosci. 2004. PMID: 15548668 Free PMC article.
-
NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus.J Cell Biol. 2004 May 24;165(4):575-89. doi: 10.1083/jcb.200311141. J Cell Biol. 2004. PMID: 15159421 Free PMC article.
-
Retrovirally delivered Islet-1 increases recruitment of Ng2 expressing cells from the postnatal SVZ into the striatum.Exp Neurol. 2006 Oct;201(2):388-98. doi: 10.1016/j.expneurol.2006.04.022. Epub 2006 Jun 27. Exp Neurol. 2006. PMID: 16806175
-
The duality of epidermal growth factor receptor (EGFR) signaling and neural stem cell phenotype: cell enhancer or cell transformer?Curr Stem Cell Res Ther. 2006 Sep;1(3):387-94. doi: 10.2174/157488806778226849. Curr Stem Cell Res Ther. 2006. PMID: 18220882 Review.
-
AN2, the mouse homologue of NG2, is a surface antigen on glial precursor cells implicated in control of cell migration.J Neurocytol. 2002 Jul-Aug;31(6-7):497-505. doi: 10.1023/a:1025743731306. J Neurocytol. 2002. PMID: 14501219 Review.
Cited by
-
N-syndecan deficiency impairs neural migration in brain.J Cell Biol. 2006 Aug 14;174(4):569-80. doi: 10.1083/jcb.200602043. J Cell Biol. 2006. PMID: 16908672 Free PMC article.
-
Cell migration in the normal and pathological postnatal mammalian brain.Prog Neurobiol. 2009 May;88(1):41-63. doi: 10.1016/j.pneurobio.2009.02.001. Epub 2009 Feb 11. Prog Neurobiol. 2009. PMID: 19428961 Free PMC article. Review.
-
Prospects and limitations of using endogenous neural stem cells for brain regeneration.Genes (Basel). 2011 Jan 14;2(1):107-30. doi: 10.3390/genes2010107. Genes (Basel). 2011. PMID: 24710140 Free PMC article.
-
Thymosin beta 4 up-regulates miR-200a expression and induces differentiation and survival of rat brain progenitor cells.J Neurochem. 2016 Jan;136(1):118-32. doi: 10.1111/jnc.13394. Epub 2015 Nov 10. J Neurochem. 2016. PMID: 26466330 Free PMC article.
-
Adult mouse subventricular zone stem and progenitor cells are sessile and epidermal growth factor receptor negatively regulates neuroblast migration.PLoS One. 2009 Dec 2;4(12):e8122. doi: 10.1371/journal.pone.0008122. PLoS One. 2009. PMID: 19956583 Free PMC article.
References
-
- Alvarez-Buylla A, Herrera DG, Wichterle H (2000) The subventricular zone: source of neuronal precursors for brain repair. Prog Brain Res 127: 1-11. - PubMed
-
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C (2004) Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci 7: 1319-1328. - PubMed
-
- Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M (2002) Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron 33: 233-248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
