Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism
- PMID: 16319222
- PMCID: PMC1312424
- DOI: 10.1073/pnas.0509201102
Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism
Abstract
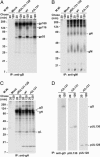
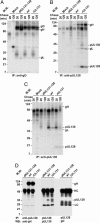
Human cytomegalovirus replicates in many different cell types, including epithelial cells, endothelial cells, and fibroblasts. However, laboratory strains of the virus, many of which were developed as attenuated vaccine candidates by serial passage in fibroblasts, have lost the ability to infect epithelial and endothelial cells. Their growth is restricted primarily to fibroblasts, due to mutations in the UL131-UL128 locus. We now demonstrate that two products of this locus, pUL130 and pUL128, form a complex with gH and gL, but not gO. The AD169 laboratory strain, which lacks a functional UL131 protein, produces virions containing only the gH-gL-gO complex. An epithelial and endothelial cell tropic AD169 variant in which the UL131 ORF has been repaired, termed BADrUL131, produces virions that carry both gH-gL-gO and gH-gL-pUL128-pUL130 complexes. Antibodies against pUL130 and pUL128 block infection of epithelial and endothelial cells by BADrUL131 and the fusion-inducing factor X clinical human cytomegalovirus isolate but do not affect the efficiency with which fibroblasts are infected.
Figures



Similar articles
-
A human cytomegalovirus gO-null mutant fails to incorporate gH/gL into the virion envelope and is unable to enter fibroblasts and epithelial and endothelial cells.J Virol. 2010 Mar;84(5):2585-96. doi: 10.1128/JVI.02249-09. Epub 2009 Dec 23. J Virol. 2010. PMID: 20032184 Free PMC article.
-
Human cytomegalovirus TR strain glycoprotein O acts as a chaperone promoting gH/gL incorporation into virions but is not present in virions.J Virol. 2010 Mar;84(5):2597-609. doi: 10.1128/JVI.02256-09. Epub 2009 Dec 23. J Virol. 2010. PMID: 20032193 Free PMC article.
-
Human Cytomegalovirus gH/gL/gO Promotes the Fusion Step of Entry into All Cell Types, whereas gH/gL/UL128-131 Broadens Virus Tropism through a Distinct Mechanism.J Virol. 2015 Sep;89(17):8999-9009. doi: 10.1128/JVI.01325-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085146 Free PMC article.
-
Principles for studying in vivo attenuation of virus mutants: defining the role of the cytomegalovirus gH/gL/gO complex as a paradigm.Med Microbiol Immunol. 2015 Jun;204(3):295-305. doi: 10.1007/s00430-015-0405-2. Epub 2015 Mar 18. Med Microbiol Immunol. 2015. PMID: 25782576 Review.
-
Human cytomegalovirus tropism for endothelial/epithelial cells: scientific background and clinical implications.Rev Med Virol. 2010 May;20(3):136-55. doi: 10.1002/rmv.645. Rev Med Virol. 2010. PMID: 20084641 Review.
Cited by
-
The proteome of human cytomegalovirus virions and dense bodies is conserved across different strains.Med Microbiol Immunol. 2015 Jun;204(3):285-93. doi: 10.1007/s00430-015-0397-y. Epub 2015 Mar 3. Med Microbiol Immunol. 2015. PMID: 25732096
-
Dissecting the cytomegalovirus CC chemokine: Chemokine activity and gHgLchemokine-dependent cell tropism are independent players in CMV infection.PLoS Pathog. 2023 Dec 8;19(12):e1011793. doi: 10.1371/journal.ppat.1011793. eCollection 2023 Dec. PLoS Pathog. 2023. PMID: 38064525 Free PMC article.
-
Human Cytomegalovirus mRNA-1647 Vaccine Candidate Elicits Potent and Broad Neutralization and Higher Antibody-Dependent Cellular Cytotoxicity Responses Than the gB/MF59 Vaccine.J Infect Dis. 2024 Aug 16;230(2):455-466. doi: 10.1093/infdis/jiad593. J Infect Dis. 2024. PMID: 38324766
-
Impact of sequence variation in the UL128 locus on production of human cytomegalovirus in fibroblast and epithelial cells.J Virol. 2013 Oct;87(19):10489-500. doi: 10.1128/JVI.01546-13. Epub 2013 Jul 24. J Virol. 2013. PMID: 23885075 Free PMC article.
-
A Homolog Pentameric Complex Dictates Viral Epithelial Tropism, Pathogenicity and Congenital Infection Rate in Guinea Pig Cytomegalovirus.PLoS Pathog. 2016 Jul 7;12(7):e1005755. doi: 10.1371/journal.ppat.1005755. eCollection 2016 Jul. PLoS Pathog. 2016. PMID: 27387220 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

