Potent antiviral activity of north-methanocarbathymidine against Kaposi's sarcoma-associated herpesvirus
- PMID: 16304159
- PMCID: PMC1315933
- DOI: 10.1128/AAC.49.12.4965-4973.2005
Potent antiviral activity of north-methanocarbathymidine against Kaposi's sarcoma-associated herpesvirus
Abstract
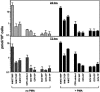
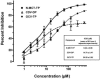
Kaposi's sarcoma-associated herpesvirus (KSHV) infection is a prerequisite for the development of Kaposi's sarcoma (KS). Blocking lytic KSHV replication may hinder KS tumorigenesis. Here, we report potent in vitro anti-KSHV activity of 2'-exo-methanocarbathymidine [North-methanocarbathymidine (N-MCT)], a thymidine analog with a pseudosugar ring locked in the northern conformation, which has previously been shown to block the replication of herpes simplex virus types 1 and 2. N-MCT inhibited KSHV virion production in lytically induced KSHV-infected BCBL-1 cells with a substantially lower 50% inhibitory concentration (IC50) than those of cidofovir (CDV) and ganciclovir (GCV) (IC50, mean +/- standard deviation: 0.08 +/- 0.03, 0.42 +/- 0.07, and 0.96 +/- 0.49 microM for N-MCT, CDV, and GCV, respectively). The reduction in KSHV virion production was accompanied by a corresponding decrease in KSHV DNA levels in the N-MCT-treated BCBL-1 cells, indicating that the compound blocked lytic KSHV DNA replication. A time- and dose-dependent accumulation of N-MCT-triphosphate (TP) was demonstrated in lytically induced BCBL-1 cells, while uninfected cells showed virtually no accumulation. The levels of N-MCT-TP were significantly decreased in the presence of 5'-ethynylthymidine, a potent inhibitor of herpesvirus thymidine kinase, resulting in the abrogation of anti-KSHV activity of N-MCT. N-MCT-TP more effectively blocked in vitro DNA synthesis by KSHV DNA polymerase with an IC50 of 6.24 +/- 0.08 microM (mean +/- standard deviation) compared to CDV-diphosphate (14.70 +/-2.47 microM) or GCV-TP (24.59 +/- 5.60 microM). Taken together, N-MCT is a highly potent and target-specific anti-KSHV agent which inhibits lytic KSHV DNA synthesis through its triphosphate metabolite produced in KSHV-infected cells expressing a virally encoded thymidine kinase.
Figures



Similar articles
-
In vitro antiviral drug sensitivity of the Kaposi's sarcoma-associated herpesvirus.AIDS. 1997 Sep;11(11):1327-32. doi: 10.1097/00002030-199711000-00006. AIDS. 1997. PMID: 9302441
-
Metabolic pathways of N-methanocarbathymidine, a novel antiviral agent, in native and herpes simplex virus type 1 infected Vero cells.Antiviral Res. 2002 Jul;55(1):63-75. doi: 10.1016/s0166-3542(02)00010-4. Antiviral Res. 2002. PMID: 12076752
-
The history of N-methanocarbathymidine: the investigation of a conformational concept leads to the discovery of a potent and selective nucleoside antiviral agent.Antiviral Res. 2006 Sep;71(2-3):268-75. doi: 10.1016/j.antiviral.2006.04.012. Epub 2006 May 6. Antiviral Res. 2006. PMID: 16730077 Review.
-
Identification of new antiviral agents against Kaposi's sarcoma-associated herpesvirus (KSHV) by high-throughput drug screening reveals the role of histamine-related signaling in promoting viral lytic reactivation.PLoS Pathog. 2019 Dec 2;15(12):e1008156. doi: 10.1371/journal.ppat.1008156. eCollection 2019 Dec. PLoS Pathog. 2019. PMID: 31790497 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus: the role of lytic replication in targeted therapy.Curr Opin Infect Dis. 2015 Dec;28(6):611-24. doi: 10.1097/QCO.0000000000000213. Curr Opin Infect Dis. 2015. PMID: 26524334 Review.
Cited by
-
Highlights in antiviral drug research: antivirals at the horizon.Med Res Rev. 2013 Nov;33(6):1215-48. doi: 10.1002/med.21256. Epub 2012 May 2. Med Res Rev. 2013. PMID: 22553111 Free PMC article. Review.
-
Activity and mechanism of action of N-methanocarbathymidine against herpesvirus and orthopoxvirus infections.Antimicrob Agents Chemother. 2006 Apr;50(4):1336-41. doi: 10.1128/AAC.50.4.1336-1341.2006. Antimicrob Agents Chemother. 2006. PMID: 16569849 Free PMC article.
-
Recent Advances in Developing Treatments of Kaposi's Sarcoma Herpesvirus-Related Diseases.Viruses. 2021 Sep 9;13(9):1797. doi: 10.3390/v13091797. Viruses. 2021. PMID: 34578378 Free PMC article. Review.
-
Status Presens of Antiviral Drugs And Strategies: Part I: DNA Viruses and Retroviruses.Adv Antivir Drug Des. 2007;5:1-58. doi: 10.1016/S1075-8593(06)05001-5. Epub 2007 Sep 2. Adv Antivir Drug Des. 2007. PMID: 32288472 Free PMC article. Review.
-
Stereoselective Synthesis and Investigation of Isopulegol-Based Chiral Ligands.Int J Mol Sci. 2019 Aug 19;20(16):4050. doi: 10.3390/ijms20164050. Int J Mol Sci. 2019. PMID: 31430981 Free PMC article.
References
-
- Anonymous. 1981. Kaposi's sarcoma and Pneumocystis pneumonia among homosexual men—New York City and California. Morb. Mortal. Wkly. Rep. 30:305-308. - PubMed
-
- Atkinson, M. R., M. P. Deutscher, A. Kornberg, A. F. Russell, and J. G. Moffatt. 1969. Enzymatic synthesis of deoxyribonucleic acid. XXXIV. Termination of chain growth by a 2′,3′-dideoxyribonucleotide. Biochemistry 8:4897-4904. - PubMed
-
- Balzarini, J., E. De Clercq, A. Verbruggen, D. Ayusawa, K. Shimizu, and T. Seno. 1987. Thymidylate synthase is the principal target enzyme for the cytostatic activity of (E)-5-(2-bromovinyl)-2′-deoxyuridine against murine mammary carcinoma (FM3A) cells transformed with the herpes simplex virus type 1 or type 2 thymidine kinase gene. Mol. Pharmacol. 32:410-416. - PubMed
-
- Boyer, P. L., J. G. Julias, V. E. Marquez, and S. H. Hughes. 2005. Fixed conformation nucleoside analogs effectively inhibit excision-proficient HIV-1 reverse transcriptases. J. Mol. Biol. 345:441-450. - PubMed
-
- Brutlag, D., and A. Kornberg. 1972. Enzymatic synthesis of deoxyribonucleic acid. 36. A proofreading function for the 3′ leads to 5′ exonuclease activity in deoxyribonucleic acid polymerases. J. Biol. Chem. 247:241-248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

