Screening of an Echinococcus granulosus cDNA library with IgG4 from patients with cystic echinococcosis identifies a new tegumental protein involved in the immune escape
- PMID: 16297166
- PMCID: PMC1809546
- DOI: 10.1111/j.1365-2249.2005.02939.x
Screening of an Echinococcus granulosus cDNA library with IgG4 from patients with cystic echinococcosis identifies a new tegumental protein involved in the immune escape
Abstract
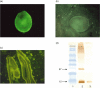
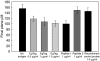
The worldwide problem of chronic Echinococcus granulosus disease calls for new parasite-derived immunomodulatory molecules. By screening an E. granulosus cDNA library with IgG4 from patients with active cystic echinococcosis, we identified a cDNA that encodes a predicted partial protein that immunofluorescence studies localized in the protoscolex tegument and on the germinal layer of cyst wall. We named this protein EgTeg because the 105 amino acid sequence scored highest against a family of Schistosoma tegumental proteins. Evaluating the role of EgTeg in the human early inflammatory response we found that EgTeg significantly inhibited polymorphonuclear cell (PMN) chemotaxis. Cytometric analysis of intracellular cytokines disclosed a significantly higher percentage of cells producing IL-4 than IFN-gamma (P = 0.001, Student's t-test) in T lymphocytes from patients with cystic echinococcosis stimulated with EgTeg. EgTeg induced weak Th1-dependent proliferation in 42% of patients' peripheral blood mononuclear cells. In immunoblotting (IB) analysis of total IgG and IgG subclass responses to EgTeg in patients with cystic echinococcosis, patients with other parasitoses, patients with cystic lesions and healthy controls, total IgG specific to EgTeg yielded high sensitivity (73%) but low specificity (44%) precluding its use in immunodiagnosis. Conversely, IgG4 specific to EgTeg gave acceptable sensitivity (65%) and high specificity (89%) suggesting its use in immunodiagnosis to confirm ultrasound documented cysts suggestive of E. granulosus. Because the new tegumental antigen EgTeg inhibits chemotaxis, induces IL-4-positive T lymphocytes and noncomplement fixing antibodies (IgG4) it is an immunomodulatory molecule associated with chronic infection.
Figures




Similar articles
-
Multiplex cytokine and antibody profile in cystic echinococcosis patients during a three-year follow-up in reference to the cyst stages.Parasit Vectors. 2020 Mar 14;13(1):133. doi: 10.1186/s13071-020-4003-9. Parasit Vectors. 2020. PMID: 32171321 Free PMC article.
-
Immunological characterization of Echinococcus granulosus cyclophilin, an allergen reactive with IgE and IgG4 from patients with cystic echinococcosis.Clin Exp Immunol. 2002 Apr;128(1):124-30. doi: 10.1046/j.1365-2249.2002.01807.x. Clin Exp Immunol. 2002. PMID: 11982600 Free PMC article.
-
Identification of a novel 19 kDa Echinococcus granulosus antigen.Acta Trop. 2010 Jan;113(1):42-7. doi: 10.1016/j.actatropica.2009.09.003. Epub 2009 Sep 19. Acta Trop. 2010. PMID: 19769934
-
Critical points in the immunodiagnosis of cystic echinococcosis in humans.Parassitologia. 2004 Dec;46(4):401-3. Parassitologia. 2004. PMID: 16044700 Review.
-
Immunomodulatory mechanisms during Echinococcus granulosus infection.Exp Parasitol. 2008 Aug;119(4):483-489. doi: 10.1016/j.exppara.2008.01.016. Epub 2008 Feb 8. Exp Parasitol. 2008. PMID: 18329023 Review.
Cited by
-
Host-parasite relationship in cystic echinococcosis: an evolving story.Clin Dev Immunol. 2012;2012:639362. doi: 10.1155/2012/639362. Epub 2011 Oct 31. Clin Dev Immunol. 2012. PMID: 22110535 Free PMC article. Review.
-
Design of a Novel Multi-Epitope Vaccine Against Echinococcus granulosus in Immunoinformatics.Front Immunol. 2021 Aug 12;12:668492. doi: 10.3389/fimmu.2021.668492. eCollection 2021. Front Immunol. 2021. PMID: 34456902 Free PMC article.
-
Transcriptome profiles of the protoscoleces of Echinococcus granulosus reveal that excretory-secretory products are essential to metabolic adaptation.PLoS Negl Trop Dis. 2014 Dec 11;8(12):e3392. doi: 10.1371/journal.pntd.0003392. eCollection 2014 Dec. PLoS Negl Trop Dis. 2014. PMID: 25500817 Free PMC article.
-
Granulocytes in helminth infection -- who is calling the shots?Curr Med Chem. 2012;19(10):1567-86. doi: 10.2174/092986712799828337. Curr Med Chem. 2012. PMID: 22360486 Free PMC article. Review.
-
Echinococcus granulosus antigen B impairs human dendritic cell differentiation and polarizes immature dendritic cell maturation towards a Th2 cell response.Infect Immun. 2007 Apr;75(4):1667-78. doi: 10.1128/IAI.01156-06. Epub 2007 Jan 8. Infect Immun. 2007. PMID: 17210662 Free PMC article.
References
-
- McManus DP, Thompson RC. Molecular epidemiology of cystic echinococcosis. Parasitology. 2003;127:S37–51. - PubMed
-
- McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;362:1295–304. - PubMed
-
- Craig PS. Immunodiagnosis of Echinococcus granulosus and a comparison of techniques for diagnosis of canine echinococcosis. In: Andersen FL, Ouhelli H, Kachani M, editors. Compendium on Cystic Echinococcosis. Provo: Brigham Young University Print Services; 1997. pp. 85–118.
-
- Ioppolo S, Notargiacomo S, Profumo E, Franchi C, Ortona E, Riganò R, Siracusano A. Immunological responses to antigen B from Echinococcus granulosus cyst fluid in hydatid patients. Parasite Immunol. 1996;18:571–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

