The antioxidant function of the p53 tumor suppressor
- PMID: 16286925
- PMCID: PMC2637821
- DOI: 10.1038/nm1320
The antioxidant function of the p53 tumor suppressor
Abstract
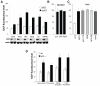
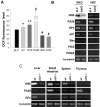
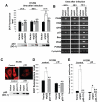
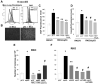
It is widely accepted that the p53 tumor suppressor restricts abnormal cells by induction of growth arrest or by triggering apoptosis. Here we show that, in addition, p53 protects the genome from oxidation by reactive oxygen species (ROS), a major cause of DNA damage and genetic instability. In the absence of severe stresses, relatively low levels of p53 are sufficient for upregulation of several genes with antioxidant products, which is associated with a decrease in intracellular ROS. Downregulation of p53 results in excessive oxidation of DNA, increased mutation rate and karyotype instability, which are prevented by incubation with the antioxidant N-acetylcysteine (NAC). Dietary supplementation with NAC prevented frequent lymphomas characteristic of Trp53-knockout mice, and slowed the growth of lung cancer xenografts deficient in p53. Our results provide a new paradigm for a nonrestrictive tumor suppressor function of p53 and highlight the potential importance of antioxidants in the prophylaxis and treatment of cancer.
Figures

Comment in
-
Savior and slayer: the two faces of p53.Nat Med. 2005 Dec;11(12):1278-9. doi: 10.1038/nm1205-1278. Nat Med. 2005. PMID: 16333263 No abstract available.
Similar articles
-
Savior and slayer: the two faces of p53.Nat Med. 2005 Dec;11(12):1278-9. doi: 10.1038/nm1205-1278. Nat Med. 2005. PMID: 16333263 No abstract available.
-
Ribosomal protein S27-like, a p53-inducible modulator of cell fate in response to genotoxic stress.Cancer Res. 2007 Dec 1;67(23):11317-26. doi: 10.1158/0008-5472.CAN-07-1088. Cancer Res. 2007. PMID: 18056458
-
Indole-3-carbinol mediated cell cycle arrest of LNCaP human prostate cancer cells requires the induced production of activated p53 tumor suppressor protein.Biochem Pharmacol. 2006 Dec 15;72(12):1714-23. doi: 10.1016/j.bcp.2006.08.012. Epub 2006 Sep 12. Biochem Pharmacol. 2006. PMID: 16970927
-
ROS and p53: a versatile partnership.Free Radic Biol Med. 2008 Apr 15;44(8):1529-35. doi: 10.1016/j.freeradbiomed.2008.01.011. Epub 2008 Jan 26. Free Radic Biol Med. 2008. PMID: 18275858 Free PMC article. Review.
-
Regulation of apoptosis by viral gene products.J Virol. 1997 Mar;71(3):1739-46. doi: 10.1128/JVI.71.3.1739-1746.1997. J Virol. 1997. PMID: 9032302 Free PMC article. Review. No abstract available.
Cited by
-
Value of serum and follicular fluid sirtuin (SIRT)1 and SIRT2 protein levels in predicting the outcome of assisted reproduction.Ann Transl Med. 2021 Feb;9(4):343. doi: 10.21037/atm-21-63. Ann Transl Med. 2021. PMID: 33708970 Free PMC article.
-
p53 Integrates host defense and cell fate during bacterial pneumonia.J Exp Med. 2013 May 6;210(5):891-904. doi: 10.1084/jem.20121674. Epub 2013 Apr 29. J Exp Med. 2013. PMID: 23630228 Free PMC article.
-
RNA-protein interaction mapping via MS2- or Cas13-based APEX targeting.Proc Natl Acad Sci U S A. 2020 Sep 8;117(36):22068-22079. doi: 10.1073/pnas.2006617117. Epub 2020 Aug 24. Proc Natl Acad Sci U S A. 2020. PMID: 32839320 Free PMC article.
-
Allele-specific p53 mutant reactivation.Cancer Cell. 2012 May 15;21(5):614-625. doi: 10.1016/j.ccr.2012.03.042. Cancer Cell. 2012. PMID: 22624712 Free PMC article.
-
Oxidative Stress Mediates the Antiproliferative Effects of Nelfinavir in Breast Cancer Cells.PLoS One. 2016 Jun 9;11(6):e0155970. doi: 10.1371/journal.pone.0155970. eCollection 2016. PLoS One. 2016. PMID: 27280849 Free PMC article.
References
-
- Lane DP. p53, guardian of the genome. Nature. 1992;358:15–16. - PubMed
-
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. - PubMed
-
- Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. - PubMed
-
- Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001;477:7–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

