CD8+ and CD20+ lymphocytes cooperate to control acute simian immunodeficiency virus/human immunodeficiency virus chimeric virus infections in rhesus monkeys: modulation by major histocompatibility complex genotype
- PMID: 16282488
- PMCID: PMC1287589
- DOI: 10.1128/JVI.79.23.14887-14898.2005
CD8+ and CD20+ lymphocytes cooperate to control acute simian immunodeficiency virus/human immunodeficiency virus chimeric virus infections in rhesus monkeys: modulation by major histocompatibility complex genotype
Abstract
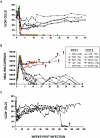
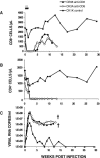
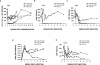
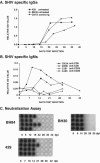
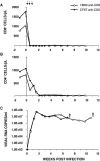
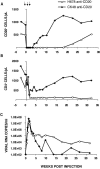
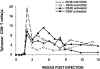
We have previously described two isogenic molecularly cloned simian immunodeficiency virus/human immunodeficiency virus chimeric viruses (SHIVs) that differ from one another by 9 amino acids and direct distinct clinical outcomes in inoculated rhesus monkeys. SHIV(DH12R-Clone 7), like other highly pathogenic CXCR4-tropic SHIVs, induces rapid and complete depletions of CD4+ T lymphocytes and immunodeficiency in infected animals. In contrast, macaques inoculated with SHIV(DH12R-Clone 8) experience only partial and transient losses of CD4+ T cells, show prompt control of their viremia, and remain healthy for periods of time extending for up to 4 years. The contributions of CD8+ and CD20+ lymphocytes in suppressing the replication of the attenuated SHIV(DH12R-Clone 8) and maintaining a prolonged asymptomatic clinical course was assessed by treating animals with monoclonal antibodies that deplete each lymphocyte subset at the time of virus inoculation. The absence of either CD8+ or CD20+ cells during the SHIV(DH12R-Clone 8) acute infection resulted in the rapid, complete, and irreversible loss of CD4+ T cells; sustained high levels of postpeak plasma viremia; and symptomatic disease in Mamu-A*01-negative Indian rhesus monkeys. In Mamu-A*01-positive animals, however, the aggressive, highly pathogenic phenotype was observed only in macaques depleted of CD8+ cells; SHIV(DH12R-Clone 8) was effectively controlled in Mamu-A*01-positive monkeys in the absence of B lymphocytes. Taken together, these results indicate that both CD8+ and CD20+ B cells contribute to the control of primate lentiviral infection in Mamu-A*01-negative macaques. Furthermore, the major histocompatibility complex genotype of an infected animal, as exemplified by the Mamu-A*01 allele in this study, has the additional capacity to shift the balance of the composite immune response.
Figures
Similar articles
-
Short- and long-term clinical outcomes in rhesus monkeys inoculated with a highly pathogenic chimeric simian/human immunodeficiency virus.J Virol. 2000 Aug;74(15):6935-45. doi: 10.1128/jvi.74.15.6935-6945.2000. J Virol. 2000. PMID: 10888632 Free PMC article.
-
V3 loop-determined coreceptor preference dictates the dynamics of CD4+-T-cell loss in simian-human immunodeficiency virus-infected macaques.J Virol. 2005 Oct;79(19):12296-303. doi: 10.1128/JVI.79.19.12296-12303.2005. J Virol. 2005. PMID: 16160156 Free PMC article.
-
Highly pathogenic SHIVs and SIVs target different CD4+ T cell subsets in rhesus monkeys, explaining their divergent clinical courses.Proc Natl Acad Sci U S A. 2004 Aug 17;101(33):12324-9. doi: 10.1073/pnas.0404620101. Epub 2004 Aug 5. Proc Natl Acad Sci U S A. 2004. PMID: 15297611 Free PMC article.
-
Understanding the basis of CD4(+) T-cell depletion in macaques infected by a simian-human immunodeficiency virus.Vaccine. 2002 May 6;20(15):1934-7. doi: 10.1016/s0264-410x(02)00072-5. Vaccine. 2002. PMID: 11983249 Review.
-
CD8+ T cell mediated noncytolytic inhibition of human immunodeficiency virus type I.Front Biosci. 2001 Mar 1;6:D575-98. doi: 10.2741/tomaras. Front Biosci. 2001. PMID: 11229882 Review.
Cited by
-
CD4+ T cells support production of simian immunodeficiency virus Env antibodies that enforce CD4-dependent entry and shape tropism in vivo.J Virol. 2013 Sep;87(17):9719-32. doi: 10.1128/JVI.01254-13. Epub 2013 Jul 3. J Virol. 2013. PMID: 23824793 Free PMC article.
-
In situ detection of Gag-specific CD8+ cells in the GI tract of SIV infected Rhesus macaques.Retrovirology. 2010 Feb 16;7:12. doi: 10.1186/1742-4690-7-12. Retrovirology. 2010. PMID: 20158906 Free PMC article.
-
Neutralizing polyclonal IgG present during acute infection prevents rapid disease onset in simian-human immunodeficiency virus SHIVSF162P3-infected infant rhesus macaques.J Virol. 2013 Oct;87(19):10447-59. doi: 10.1128/JVI.00049-13. Epub 2013 Jul 24. J Virol. 2013. PMID: 23885083 Free PMC article.
-
Effect of B-cell depletion on coreceptor switching in R5 simian-human immunodeficiency virus infection of rhesus macaques.J Virol. 2011 Apr;85(7):3086-94. doi: 10.1128/JVI.02150-10. Epub 2011 Jan 19. J Virol. 2011. PMID: 21248033 Free PMC article.
-
Microbial TLR Agonists and Humoral Immunopathogenesis in HIV Disease.Epidemiology (Sunnyvale). 2013 Feb 2;3:120. doi: 10.4172/2161-1165.1000120. Epidemiology (Sunnyvale). 2013. PMID: 24795844 Free PMC article.
References
-
- Aasa-Chapman, M. M., S. Holuigue, K. Aubin, M. Wong, N. A. Jones, D. Cornforth, P. Pellegrino, P. Newton, I. Williams, P. Borrow, and A. McKnight. 2005. Detection of antibody-dependent complement-mediated inactivation of both autologous and heterologous virus in primary human immunodeficiency virus type 1 infection. J. Virol. 79:2823-2830. - PMC - PubMed
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. - PMC - PubMed
-
- Committee on Care and Use of Laboratory Animals. 1985. Guide for the care and use of laboratory animals. Department of Health and Human Services publication no. NIH 85-23. National Institutes of Health, Bethesda, Md.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

