Assay for high glucose-mediated islet cell sensitization to apoptosis induced by streptozotocin and cytokines
- PMID: 16281079
- PMCID: PMC1280327
- DOI: 10.1251/bpo113
Assay for high glucose-mediated islet cell sensitization to apoptosis induced by streptozotocin and cytokines
Abstract
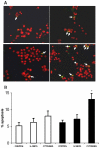
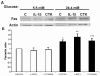
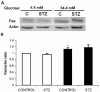
Pancreatic beta-cell apoptosis is known to participate in the beta-cell destruction process that occurs in diabetes. It has been described that high glucose level induces a hyperfunctional status which could provoke apoptosis. This phenomenon is known as glucotoxicity and has been proposed that it can play a role in type 1 diabetes mellitus pathogenesis. In this study we develop an experimental design to sensitize pancreatic islet cells by high glucose to streptozotocin (STZ) and proinflammatory cytokines [interleukin (IL)-1beta, tumor necrosis factor (TNF)-alpha and interferon (IFN)-gamma]-induced apoptosis. This method is appropriate for subsequent quantification of apoptotic islet cells stained with Tdt-mediated dUTP Nick-End Labeling (TUNEL) and protein expression assays by Western Blotting (WB).
Figures
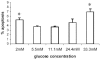
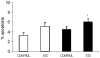


Similar articles
-
High glucose potentiates cytokine- and streptozotocin-induced apoptosis of rat islet cells: effect on apoptosis-related genes.J Endocrinol. 2004 Oct;183(1):155-62. doi: 10.1677/joe.1.05542. J Endocrinol. 2004. PMID: 15525583
-
Expression of calbindin-D(28k) in a pancreatic islet beta-cell line protects against cytokine-induced apoptosis and necrosis.Endocrinology. 2001 Aug;142(8):3649-55. doi: 10.1210/endo.142.8.8334. Endocrinology. 2001. PMID: 11459814
-
STAT5 activation by human GH protects insulin-producing cells against interleukin-1beta, interferon-gamma and tumour necrosis factor-alpha-induced apoptosis independent of nitric oxide production.J Endocrinol. 2005 Oct;187(1):25-36. doi: 10.1677/joe.1.06086. J Endocrinol. 2005. PMID: 16214938
-
An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus.Diabetes Metab Rev. 1998 Jun;14(2):129-51. doi: 10.1002/(sici)1099-0895(199806)14:2<129::aid-dmr208>3.0.co;2-v. Diabetes Metab Rev. 1998. PMID: 9679667 Review.
-
Role of pro-inflammatory cytokines of pancreatic islets and prospects of elaboration of new methods for the diabetes treatment.Acta Biochim Pol. 2015;62(1):15-21. doi: 10.18388/abp.2014_853. Epub 2015 Mar 18. Acta Biochim Pol. 2015. PMID: 25781159 Review.
Cited by
-
Toward closing the loop: an update on insulin pumps and continuous glucose monitoring systems.Endocrinol Metab Clin North Am. 2010 Sep;39(3):609-24. doi: 10.1016/j.ecl.2010.05.005. Endocrinol Metab Clin North Am. 2010. PMID: 20723823 Free PMC article. Review.
-
Epigallocatechin gallate attenuated high glucose-induced pancreatic beta cell dysfunction by modulating DRP1-mediated mitochondrial apoptosis pathways.Sci Rep. 2024 Jul 22;14(1):16809. doi: 10.1038/s41598-024-67867-0. Sci Rep. 2024. PMID: 39039202 Free PMC article.
-
Transfection of rat pancreatic islet tissue by polymeric gene vectors.Diabetes Technol Ther. 2009 Jul;11(7):443-9. doi: 10.1089/dia.2008.0117. Diabetes Technol Ther. 2009. PMID: 19580358 Free PMC article.
-
Heat shock protein 27 overexpression mitigates cytokine-induced islet apoptosis and streptozotocin-induced diabetes.Endocrinology. 2009 Jul;150(7):3031-9. doi: 10.1210/en.2008-0732. Epub 2009 Mar 26. Endocrinology. 2009. PMID: 19325007 Free PMC article.
References
-
- O'Brien BA, Harmon BV, Cameron DP, Allan DJ. Apoptosis is the mode of [beta]-cell death responsible for the development of IDDM in the nonobese diabetic (NOD) mouse. Diabetes. 1997;46:750–757. - PubMed
-
- Kaneto H, Fujii J, Seo HG. Apoptotic cell death triggered by nitric oxide in pancreatic beta-cells. Diabetes. 1995;44:733–738. - PubMed

