TNF-alpha dilates cerebral arteries via NAD(P)H oxidase-dependent Ca2+ spark activation
- PMID: 16267103
- PMCID: PMC1638900
- DOI: 10.1152/ajpcell.00499.2005
TNF-alpha dilates cerebral arteries via NAD(P)H oxidase-dependent Ca2+ spark activation
Abstract
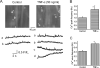
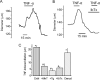
Expression of TNF-alpha, a pleiotropic cytokine, is elevated during stroke and cerebral ischemia. TNF-alpha regulates arterial diameter, although mechanisms mediating this effect are unclear. In the present study, we tested the hypothesis that TNF-alpha regulates the diameter of resistance-sized ( approximately 150-microm diameter) cerebral arteries by modulating local and global intracellular Ca(2+) signals in smooth muscle cells. Laser-scanning confocal imaging revealed that TNF-alpha increased Ca(2+) spark and Ca(2+) wave frequency but reduced global intracellular Ca(2+) concentration ([Ca(2+)](i)) in smooth muscle cells of intact arteries. TNF-alpha elevated reactive oxygen species (ROS) in smooth muscle cells of intact arteries, and this increase was prevented by apocynin or diphenyleneiodonium (DPI), both of which are NAD(P)H oxidase blockers, but was unaffected by inhibitors of other ROS-generating enzymes. In voltage-clamped (-40 mV) cells, TNF-alpha increased the frequency and amplitude of Ca(2+) spark-induced, large-conductance, Ca(2+)-activated K(+) (K(Ca)) channel transients approximately 1.7- and approximately 1.4-fold, respectively. TNF-alpha-induced transient K(Ca) current activation was reversed by apocynin or by Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP), a membrane-permeant antioxidant, and was prevented by intracellular dialysis of catalase. TNF-alpha induced reversible and similar amplitude dilations in either endothelium-intact or endothelium-denuded pressurized (60 mmHg) cerebral arteries. MnTMPyP, thapsigargin, a sarcoplasmic reticulum Ca(2+)-ATPase blocker that inhibits Ca(2+) sparks, and iberiotoxin, a K(Ca) channel blocker, reduced TNF-alpha-induced vasodilations to between 15 and 33% of control. In summary, our data indicate that TNF-alpha activates NAD(P)H oxidase, resulting in an increase in intracellular H(2)O(2) that stimulates Ca(2+) sparks and transient K(Ca) currents, leading to a reduction in global [Ca(2+)](i), and vasodilation.
Figures



Comment in
-
Radicals spark interest in cerebral vasodilator mechanisms. Focus on "TNF-alpha dilates cerebral arteries via NAD(P)H oxidase-dependent Ca2+ spark activation".Am J Physiol Cell Physiol. 2006 Apr;290(4):C950-1. doi: 10.1152/ajpcell.00601.2005. Am J Physiol Cell Physiol. 2006. PMID: 16531572 No abstract available.
Similar articles
-
Mitochondrial modulation of Ca2+ sparks and transient KCa currents in smooth muscle cells of rat cerebral arteries.J Physiol. 2004 May 1;556(Pt 3):755-71. doi: 10.1113/jphysiol.2003.059568. Epub 2004 Feb 6. J Physiol. 2004. PMID: 14766935 Free PMC article.
-
Intravascular pressure regulates local and global Ca(2+) signaling in cerebral artery smooth muscle cells.Am J Physiol Cell Physiol. 2001 Aug;281(2):C439-48. doi: 10.1152/ajpcell.2001.281.2.C439. Am J Physiol Cell Physiol. 2001. PMID: 11443043
-
Hypoxia reduces KCa channel activity by inducing Ca2+ spark uncoupling in cerebral artery smooth muscle cells.Am J Physiol Cell Physiol. 2007 Jun;292(6):C2122-8. doi: 10.1152/ajpcell.00629.2006. Epub 2007 Feb 21. Am J Physiol Cell Physiol. 2007. PMID: 17314264 Free PMC article.
-
Ca2+ channels, ryanodine receptors and Ca(2+)-activated K+ channels: a functional unit for regulating arterial tone.Acta Physiol Scand. 1998 Dec;164(4):577-87. doi: 10.1046/j.1365-201X.1998.00462.x. Acta Physiol Scand. 1998. PMID: 9887980 Review.
-
Endothelium-dependent cerebral artery dilation mediated by transient receptor potential and Ca2+-activated K+ channels.J Cardiovasc Pharmacol. 2011 Feb;57(2):148-53. doi: 10.1097/FJC.0b013e3181f580d9. J Cardiovasc Pharmacol. 2011. PMID: 20729757 Review.
Cited by
-
Hydrogen sulfide activates Ca²⁺ sparks to induce cerebral arteriole dilatation.J Physiol. 2012 Jun 1;590(11):2709-20. doi: 10.1113/jphysiol.2011.225128. Epub 2012 Apr 16. J Physiol. 2012. PMID: 22508960 Free PMC article.
-
The Up-regulation of TNF-α Maintains Trigeminal Neuralgia by Modulating MAPKs Phosphorylation and BKCa Channels in Trigeminal Nucleus Caudalis.Front Cell Neurosci. 2021 Nov 25;15:764141. doi: 10.3389/fncel.2021.764141. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34899191 Free PMC article.
-
Autophagy proteins control goblet cell function by potentiating reactive oxygen species production.EMBO J. 2013 Dec 11;32(24):3130-44. doi: 10.1038/emboj.2013.233. Epub 2013 Nov 1. EMBO J. 2013. PMID: 24185898 Free PMC article.
-
Microvascular NADPH oxidase in health and disease.Free Radic Biol Med. 2017 Aug;109:33-47. doi: 10.1016/j.freeradbiomed.2017.02.049. Epub 2017 Mar 6. Free Radic Biol Med. 2017. PMID: 28274817 Free PMC article. Review.
-
Potassium Channels in Regulation of Vascular Smooth Muscle Contraction and Growth.Adv Pharmacol. 2017;78:89-144. doi: 10.1016/bs.apha.2016.07.001. Epub 2016 Aug 17. Adv Pharmacol. 2017. PMID: 28212804 Free PMC article. Review.
References
-
- Aoki N, Siegfried M, Lefer AM. Anti-EDRF effect of tumor necrosis factor in isolated, perfused cat carotid arteries. Am J Physiol. 1989;256:H1509–H1512. - PubMed
-
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-α. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. - PubMed
-
- Baudry N, Vicaut E. Role of nitric oxide in effects of tumor necrosis factor-α on microcirculation in rat. J Appl Physiol. 1993;75:2392–2399. - PubMed
-
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

