The differential role of Smad2 and Smad3 in the regulation of pro-fibrotic TGFbeta1 responses in human proximal-tubule epithelial cells
- PMID: 16253118
- PMCID: PMC1360711
- DOI: 10.1042/BJ20051106
The differential role of Smad2 and Smad3 in the regulation of pro-fibrotic TGFbeta1 responses in human proximal-tubule epithelial cells
Abstract
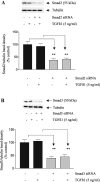
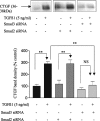
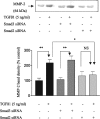
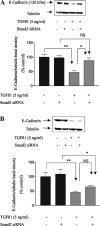
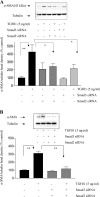
In chronic renal diseases, progressive loss of renal function correlates with advancing tubulo-interstitial fibrosis. TGFbeta1-Smad (transforming growth factor-beta1-Sma and Mad protein) signalling plays an important role in the development of renal tubulo-interstitial fibrosis. Secretion of CTGF (connective-tissue growth factor; CCN2) by PTECs (proximal-tubule epithelial cells) and EMT (epithelial-mesenchymal transdifferentiation) of PTECs to myofibroblasts in response to TGFbeta are critical Smad-dependent events in the development of tubulo-interstitial fibrosis. In the present study we have investigated the distinct contributions of Smad2 and Smad3 to expression of CTGF, E-cadherin, alpha-SMA (alpha-smooth-muscle actin) and MMP-2 (matrix-metalloproteinase-2) in response to TGFbeta1 treatment in an in vitro culture model of HKC-8 (transformed human PTECs). RNA interference was used to achieve selective and specific knockdown of Smad2 and Smad3. Cellular E-cadherin, alpha-SMA as well as secreted CTGF and MMP-2 were assessed by Western immunoblotting. TGFbeta1 treatment induced a fibrotic phenotype with increased expression of CTGF, MMP-2 and alpha-SMA, and decreased expression of E-cadherin. TGFbeta1-induced increases in CTGF and decreases in E-cadherin expression were Smad3-dependent, whereas increases in MMP-2 expression were Smad2-dependent. Increases in alpha-SMA expression were dependent on both Smad2 and Smad3 and were abolished by combined knockdown of both Smad2 and Smad3. In conclusion, we have demonstrated distinct roles for Smad2 and Smad3 in TGFbeta1-induced CTGF expression and markers of EMT in human PTECs. This can be of therapeutic value in designing targeted anti-fibrotic therapies for tubulo-interstitial fibrosis.
Figures

Similar articles
-
TGFbeta1 induces epithelial-mesenchymal transition, but not myofibroblast transdifferentiation of human kidney tubular epithelial cells in primary culture.Int J Exp Pathol. 2006 Jun;87(3):197-208. doi: 10.1111/j.1365-2613.2006.00479.x. Int J Exp Pathol. 2006. PMID: 16709228 Free PMC article.
-
TGF-β2 induces transdifferentiation and fibrosis in human lens epithelial cells via regulating gremlin and CTGF.Biochem Biophys Res Commun. 2014 May 16;447(4):689-95. doi: 10.1016/j.bbrc.2014.04.068. Epub 2014 Apr 19. Biochem Biophys Res Commun. 2014. PMID: 24755068
-
TGF-beta1-induced connective tissue growth factor (CCN2) expression in human renal proximal tubule epithelial cells requires Ras/MEK/ERK and Smad signalling.Nephron Exp Nephrol. 2005;100(4):e156-65. doi: 10.1159/000085445. Epub 2005 Apr 25. Nephron Exp Nephrol. 2005. PMID: 15855807
-
Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy?Matrix Biol. 2002 Oct;21(6):473-82. doi: 10.1016/s0945-053x(02)00055-0. Matrix Biol. 2002. PMID: 12392758 Review.
-
Therapeutic strategies to target connective tissue growth factor in fibrotic lung diseases.Pharmacol Ther. 2024 Jan;253:108578. doi: 10.1016/j.pharmthera.2023.108578. Epub 2023 Dec 15. Pharmacol Ther. 2024. PMID: 38103794 Review.
Cited by
-
Proteinase-activated receptor-2 transactivation of epidermal growth factor receptor and transforming growth factor-β receptor signaling pathways contributes to renal fibrosis.J Biol Chem. 2013 Dec 27;288(52):37319-31. doi: 10.1074/jbc.M113.492793. Epub 2013 Nov 19. J Biol Chem. 2013. PMID: 24253040 Free PMC article.
-
Fibrotic protein expression profiles in penile tissue of patients with erectile dysfunction.Urology. 2013 Oct;82(4):975.e1-6. doi: 10.1016/j.urology.2013.06.042. Urology. 2013. PMID: 24075003 Free PMC article.
-
Blocking BAFF Alleviates Hepatic Fibrosis in Schistosoma japonicum-Infected Mice.Pathogens. 2023 Jun 1;12(6):793. doi: 10.3390/pathogens12060793. Pathogens. 2023. PMID: 37375483 Free PMC article.
-
Molecular mechanisms involved in podocyte EMT and concomitant diabetic kidney diseases: an update.Ren Fail. 2017 Nov;39(1):474-483. doi: 10.1080/0886022X.2017.1313164. Ren Fail. 2017. PMID: 28413908 Free PMC article. Review.
-
Role of SARA (SMAD anchor for receptor activation) in maintenance of epithelial cell phenotype.J Biol Chem. 2009 Sep 11;284(37):25181-9. doi: 10.1074/jbc.M109.032847. Epub 2009 Jul 20. J Biol Chem. 2009. PMID: 19620243 Free PMC article.
References
-
- Nath K. A. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am. J. Kidney Dis. 1992;20:1–17. - PubMed
-
- Border W. A., Noble N. A. TGF-β in kidney fibrosis: a target for gene therapy. Kidney Int. 1997;51:1388–1396. - PubMed
-
- Bottinger E. P., Bitzer M. TGF-β signaling in renal disease. J. Am. Soc. Nephrol. 2002;13:2600–2610. - PubMed
-
- Okada H., Kikuta T., Kobayashi T., Inoue T., Kanno Y., Takigawa M., Sugaya T., Kopp J. B., Suzuki H., Kikuta T. Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J. Am. Soc. Nephrol. 2005;16:133–143. - PubMed
-
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 2004;15:1–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

