Changes in tight junctional resistance of the cervical epithelium are associated with modulation of content and phosphorylation of occludin 65-kilodalton and 50-kilodalton forms
- PMID: 16239297
- PMCID: PMC2409057
- DOI: 10.1210/en.2005-0916
Changes in tight junctional resistance of the cervical epithelium are associated with modulation of content and phosphorylation of occludin 65-kilodalton and 50-kilodalton forms
Abstract
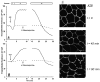
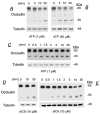
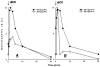
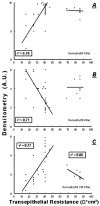
Treatment of human cervical epithelial CaSki cells with ATP or with the diacylglyceride sn-1,2-dioctanoyl diglyceride (diC8) induced a staurosporine-sensitive transient increase, followed by a late decrease, in tight-junctional resistance (R(TJ)). CaSki cells express two immunoreactive forms of occludin, 65 and 50 kDa. Treatments with ATP and diC8 decreased the density of the 65-kDa form and increased the density of the 50-kDa form. ATP also decreased threonine phosphorylation of the 65-kDa form and increased threonine phosphorylation of the 50-kDa form and tyrosine phosphorylation of the 65- and 50-kDa forms. Staurosporine decreased acutely threonine and tyrosine phosphorylation of the two isoforms and in cells pretreated with staurosporine ATP increased acutely the density of the 65-kDa form and threonine phosphorylation of the 65-kDa form. Treatment with N-acetyl-leucinyl-leucinyl-norleucinal increased the densities of the 65- and 50-kDa forms. Pretreatment with N-acetyl-leucinyl-leucinyl-norleucinal attenuated the late decreases in R(TJ) induced by ATP and diC8 and the decrease in the 65-kDa and increase in the 50-kDa forms induced by ATP. Correlation analyses showed that high levels of R(TJ) correlated with the 65-kDa form, whereas low levels of R(TJ) correlated negatively with the 65-kDa form and positively with the 50-kDa form. The results suggest that in CaSki cells 1) occludin determines gating of the tight junctions, 2) changes in occludin phosphorylation status and composition regulate the R(TJ), 3) protein kinase-C-mediated, threonine dephosphorylation of the 65-kDa occludin form increases the resistance of assembled tight junctions, 4) the early stage of tight junction disassembly involves calpain-mediated breakdown of occludin 65-kDa form to the 50-kDa form, and 5) increased levels of the 50-kDa form interfere with occludin gating of the tight junctions.
Figures






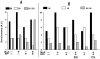

Similar articles
-
Estrogen abrogates transcervical tight junctional resistance by acceleration of occludin modulation.J Clin Endocrinol Metab. 2004 Oct;89(10):5145-55. doi: 10.1210/jc.2004-0823. J Clin Endocrinol Metab. 2004. PMID: 15472219
-
Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins.Am J Physiol. 1999 May;276(5):F737-50. doi: 10.1152/ajprenal.1999.276.5.F737. Am J Physiol. 1999. PMID: 10330056
-
Cholesterol depletion alters detergent-specific solubility profiles of selected tight junction proteins and the phosphorylation of occludin.Exp Cell Res. 2007 Jul 15;313(12):2597-610. doi: 10.1016/j.yexcr.2007.05.009. Epub 2007 May 18. Exp Cell Res. 2007. PMID: 17574235 Free PMC article.
-
Occludin: one protein, many forms.Mol Cell Biol. 2012 Jan;32(2):242-50. doi: 10.1128/MCB.06029-11. Epub 2011 Nov 14. Mol Cell Biol. 2012. PMID: 22083955 Free PMC article. Review.
-
Regulation of the epithelial barrier by post-translational modifications of tight junction membrane proteins.J Biochem. 2018 Apr 1;163(4):265-272. doi: 10.1093/jb/mvx077. J Biochem. 2018. PMID: 29186552 Review.
Cited by
-
TLR2-induced calpain cleavage of epithelial junctional proteins facilitates leukocyte transmigration.Cell Host Microbe. 2009 Jan 22;5(1):47-58. doi: 10.1016/j.chom.2008.11.009. Cell Host Microbe. 2009. PMID: 19154987 Free PMC article.
-
Occludin is regulated by epidermal growth factor receptor activation in brain endothelial cells and brains of mice with acute liver failure.Hepatology. 2011 Apr;53(4):1294-305. doi: 10.1002/hep.24161. Hepatology. 2011. PMID: 21480332 Free PMC article.
-
Ca2+ signaling in airway epithelial cells facilitates leukocyte recruitment and transepithelial migration.J Leukoc Biol. 2009 Nov;86(5):1135-44. doi: 10.1189/jlb.0209072. Epub 2009 Jul 15. J Leukoc Biol. 2009. PMID: 19605699 Free PMC article. Review.
-
The blood-brain barrier is disrupted in Machado-Joseph disease/spinocerebellar ataxia type 3: evidence from transgenic mice and human post-mortem samples.Acta Neuropathol Commun. 2020 Aug 31;8(1):152. doi: 10.1186/s40478-020-00955-0. Acta Neuropathol Commun. 2020. PMID: 32867861 Free PMC article.
-
Estrogen modulation of epithelial permeability in cervical-vaginal cells of premenopausal and postmenopausal women.Menopause. 2007 Nov-Dec;14(6):1012-9. doi: 10.1097/gme.0b013e3180587eb5. Menopause. 2007. PMID: 17572644 Free PMC article.
References
-
- Gorodeski GI. The cervical cycle. In: Adashi EY, Rock JA, Rosenwaks Z, editors. Reproductive endocrinology, surgery, and technology. Philadelphia: Lippincott-Raven; 1996. pp. 301–324.
-
- Gorodeski GI. The cultured human cervical epithelium: a new model for studying transepithelial paracellular transport. J Soc Gynecol Invest. 1996;3:267–280. - PubMed
-
- Gorodeski GI, Merlin D, De Santis BJ, Frieden KA, Hopfer U, Eckert RL, Romero MF. Characterization of paracellular permeability in cultured human cervical epithelium: regulation by extracellular ATP. J Soc Gynecol Invest. 1994;1:225–233. - PubMed
-
- Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

