The Drosophila gamma-tubulin small complex subunit Dgrip84 is required for structural and functional integrity of the spindle apparatus
- PMID: 16236791
- PMCID: PMC1345665
- DOI: 10.1091/mbc.e05-08-0722
The Drosophila gamma-tubulin small complex subunit Dgrip84 is required for structural and functional integrity of the spindle apparatus
Abstract
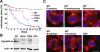
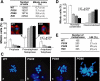
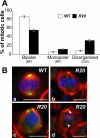
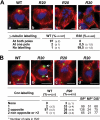
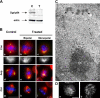
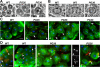
Gamma-tubulin, a protein critical for microtubule assembly, functions within multiprotein complexes. However, little is known about the respective role of gamma-tubulin partners in metazoans. For the first time in a multicellular organism, we have investigated the function of Dgrip84, the Drosophila orthologue of the Saccharomyces cerevisiae gamma-tubulin-associated protein Spc97p. Mutant analysis shows that Dgrip84 is essential for viability. Its depletion promotes a moderate increase in the mitotic index, correlated with the appearance of monopolar or unpolarized spindles, impairment of centrosome maturation, and increase of polyploid nuclei. This in vivo study is strengthened by an RNA interference approach in cultured S2 cells. Electron microscopy analysis suggests that monopolar spindles might result from a failure of centrosome separation and an unusual microtubule assembly pathway via centriolar triplets. Moreover, we point to an involvement of Dgrip84 in the spindle checkpoint regulation and in the maintenance of interphase microtubule dynamics. Dgrip84 also seems essential for male meiosis, ensuring spindle bipolarity and correct completion of cytokinesis. These data sustain that Dgrip84 is required in some aspects of microtubule dynamics and organization both in interphase and mitosis. The nature of a minimal gamma-tubulin complex necessary for proper microtubule organization in the metazoans is discussed.
Figures
Similar articles
-
Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle.J Cell Biol. 2008 May 5;181(3):421-9. doi: 10.1083/jcb.200711053. Epub 2008 Apr 28. J Cell Biol. 2008. PMID: 18443220 Free PMC article.
-
Elongation of centriolar microtubule triplets contributes to the formation of the mitotic spindle in gamma-tubulin-depleted cells.J Cell Sci. 2004 Nov 1;117(Pt 23):5497-507. doi: 10.1242/jcs.01401. Epub 2004 Oct 12. J Cell Sci. 2004. PMID: 15479719
-
A centrosome-independent role for gamma-TuRC proteins in the spindle assembly checkpoint.Science. 2006 Oct 27;314(5799):654-7. doi: 10.1126/science.1132834. Science. 2006. PMID: 17068266
-
Gamma-tubulin at ten: progress and prospects.Cell Struct Funct. 1999 Oct;24(5):365-72. doi: 10.1247/csf.24.365. Cell Struct Funct. 1999. PMID: 15216894 Review.
-
Targeting of γ-tubulin complexes to microtubule organizing centers: conservation and divergence.Trends Cell Biol. 2015 May;25(5):296-307. doi: 10.1016/j.tcb.2014.12.002. Epub 2014 Dec 24. Trends Cell Biol. 2015. PMID: 25544667 Review.
Cited by
-
LGALS3BP regulates centriole biogenesis and centrosome hypertrophy in cancer cells.Nat Commun. 2013;4:1531. doi: 10.1038/ncomms2517. Nat Commun. 2013. PMID: 23443559
-
The GCP3-interacting proteins GIP1 and GIP2 are required for γ-tubulin complex protein localization, spindle integrity, and chromosomal stability.Plant Cell. 2012 Mar;24(3):1171-87. doi: 10.1105/tpc.111.094904. Epub 2012 Mar 16. Plant Cell. 2012. PMID: 22427335 Free PMC article.
-
Centrosomal and Non-Centrosomal Microtubule-Organizing Centers (MTOCs) in Drosophila melanogaster.Cells. 2018 Aug 28;7(9):121. doi: 10.3390/cells7090121. Cells. 2018. PMID: 30154378 Free PMC article. Review.
-
Versatile gamma-tubulin complexes contribute to the dynamic organization of MTOCs during Drosophila spermatogenesis.Commun Biol. 2024 Oct 24;7(1):1385. doi: 10.1038/s42003-024-07090-9. Commun Biol. 2024. PMID: 39448788 Free PMC article.
-
A novel microtubule nucleation pathway for meiotic spindle assembly in oocytes.J Cell Biol. 2018 Oct 1;217(10):3431-3445. doi: 10.1083/jcb.201803172. Epub 2018 Aug 7. J Cell Biol. 2018. PMID: 30087124 Free PMC article.
References
-
- Barbosa, V., Gatt, M., Rebollo, E., Gonzalez, C., and Glover, S. M. (2003). Drosophila dd4 mutants reveal that γTuRC is required to maintain juxtaposed half spindles in spermatocytes. J. Cell Sci. 116, 929–941. - PubMed
-
- Bobinnec, Y., Fukuda, M., and Nishida, E. (2000). Identification and characterization of Caenorhabditis elegans γ-tubulin in dividing cells and differentiated tissues. J. Cell Sci. 113, 3747–3759. - PubMed
-
- Bonaccorsi, S., Giansanti, M. G., and Gatti, M. (2000). Spindle assembly in Drosophila neuroblasts and ganglion mother cells. Nat. Cell Biol. 2, 54–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

