Long-chain base kinase Lcb4 Is anchored to the membrane through its palmitoylation by Akr1
- PMID: 16227572
- PMCID: PMC1265802
- DOI: 10.1128/MCB.25.21.9189-9197.2005
Long-chain base kinase Lcb4 Is anchored to the membrane through its palmitoylation by Akr1
Abstract
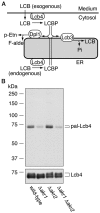
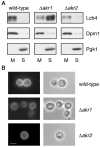
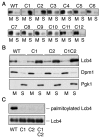
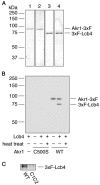
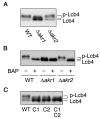
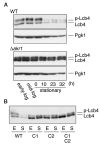
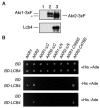
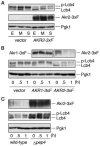
Sphingoid long-chain base kinase Lcb4 catalyzes the production of the bioactive lipid molecules the long-chain base 1-phosphates. Although Lcb4 has no apparent transmembrane-spanning domain, it is tightly associated with the membrane. Here, we demonstrate that Lcb4 is modified by palmitoylation. This modification was greatly reduced in mutants for AKR1, which was recently identified as encoding a protein acyltransferase. In vitro experiments revealed that Akr1 indeed acts as a protein acyltransferase for Lcb4. Studies using site-directed mutagenesis indicated that Cys-43 and Cys-46 are palmitoylated. The loss of palmitoylation on Lcb4 caused several effects, including mislocalization of the protein to the cytosol, reduced phosphorylation, and loss of downregulation during the stationary phase. Although Akr2 is highly homologous to Akr1, the deletion of AKR2 did not result in any remarkable phenotypes. However, overproduction of Akr2 resulted in reduced amounts of Lcb4. We demonstrated that Akr2 is an unstable protein and is degraded in the vacuole. Akr2 exhibits high affinity for Lcb4, and in Akr2-overproducing cells this interaction caused unusual delivery of Lcb4 to the vacuole and degradation.
Figures
Similar articles
-
Intracellular trafficking pathway of yeast long-chain base kinase Lcb4, from its synthesis to its degradation.J Biol Chem. 2007 Sep 28;282(39):28485-28492. doi: 10.1074/jbc.M701607200. Epub 2007 Aug 8. J Biol Chem. 2007. PMID: 17686782
-
Phosphorylation by Pho85 cyclin-dependent kinase acts as a signal for the down-regulation of the yeast sphingoid long-chain base kinase Lcb4 during the stationary phase.J Biol Chem. 2005 Feb 25;280(8):6520-7. doi: 10.1074/jbc.M410908200. Epub 2004 Dec 14. J Biol Chem. 2005. PMID: 15598647
-
The LCB4 (YOR171c) and LCB5 (YLR260w) genes of Saccharomyces encode sphingoid long chain base kinases.J Biol Chem. 1998 Jul 31;273(31):19437-42. doi: 10.1074/jbc.273.31.19437. J Biol Chem. 1998. PMID: 9677363
-
Probing protein palmitoylation at the yeast vacuole.Methods. 2006 Oct;40(2):171-6. doi: 10.1016/j.ymeth.2006.06.020. Methods. 2006. PMID: 17012029 Review.
-
Protein palmitoylation by a family of DHHC protein S-acyltransferases.J Lipid Res. 2006 Jun;47(6):1118-27. doi: 10.1194/jlr.R600007-JLR200. Epub 2006 Apr 1. J Lipid Res. 2006. PMID: 16582420 Review.
Cited by
-
Analysis of substrate specificity of human DHHC protein acyltransferases using a yeast expression system.Mol Biol Cell. 2012 Dec;23(23):4543-51. doi: 10.1091/mbc.E12-05-0336. Epub 2012 Oct 3. Mol Biol Cell. 2012. PMID: 23034182 Free PMC article.
-
Knockout of the S-acyltransferase Gene, PbPAT14, Confers the Dwarf Yellowing Phenotype in First Generation Pear by ABA Accumulation.Int J Mol Sci. 2019 Dec 16;20(24):6347. doi: 10.3390/ijms20246347. Int J Mol Sci. 2019. PMID: 31888281 Free PMC article.
-
Phytosphingosine degradation pathway includes fatty acid α-oxidation reactions in the endoplasmic reticulum.Proc Natl Acad Sci U S A. 2017 Mar 28;114(13):E2616-E2623. doi: 10.1073/pnas.1700138114. Epub 2017 Mar 13. Proc Natl Acad Sci U S A. 2017. PMID: 28289220 Free PMC article.
-
Yeast sphingolipids: recent developments in understanding biosynthesis, regulation, and function.Biochim Biophys Acta. 2007 Mar;1771(3):421-31. doi: 10.1016/j.bbalip.2006.08.005. Epub 2006 Aug 10. Biochim Biophys Acta. 2007. PMID: 16997623 Free PMC article. Review.
-
Yeast Mpo1 Is a Novel Dioxygenase That Catalyzes the α-Oxidation of a 2-Hydroxy Fatty Acid in an Fe2+-Dependent Manner.Mol Cell Biol. 2019 Feb 15;39(5):e00428-18. doi: 10.1128/MCB.00428-18. Print 2019 Mar 1. Mol Cell Biol. 2019. PMID: 30530523 Free PMC article.
References
-
- Babu, P., R. J. Deschenes, and L. C. Robinson. 2004. Akr1p-dependent palmitoylation of Yck2p yeast casein kinase 1 is necessary and sufficient for plasma membrane targeting. J. Biol. Chem. 279:27138-27147. - PubMed
-
- Birchwood, C. J., J. D. Saba, R. C. Dickson, and K. W. Cunningham. 2001. Calcium influx and signaling in yeast stimulated by intracellular sphingosine 1-phosphate accumulation. J. Biol. Chem. 276:11712-11718. - PubMed
-
- Casey, P. J. 1995. Protein lipidation in cell signaling. Science 268:221-225. - PubMed
-
- Christianson, T. W., R. S. Sikorski, M. Dante, and P. Hieter. 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110:119-122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
