Human immunodeficiency virus type 1 Vpr interacts with antiapoptotic mitochondrial protein HAX-1
- PMID: 16227293
- PMCID: PMC1262574
- DOI: 10.1128/JVI.79.21.13735-13746.2005
Human immunodeficiency virus type 1 Vpr interacts with antiapoptotic mitochondrial protein HAX-1
Abstract
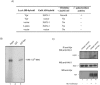
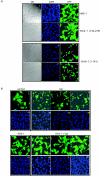
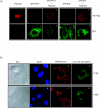
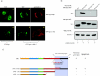
Human immunodeficiency virus type 1 viral protein R (Vpr) is required for viral pathogenesis and has been implicated in T-cell apoptosis through its activation of caspase 3 and caspase 9 and perturbation of mitochondrial membrane potential. To understand better Vpr-mitochondria interaction, we report here the identification of antiapoptotic mitochondrial protein HAX-1 as a novel Vpr target. We show that Vpr and HAX-1 physically associate with each other. Overexpression of Vpr in cells dislocates HAX-1 from its normal residence in mitochondria and creates mitochondrion instability and cell death. Conversely, overexpression of HAX-1 suppressed the proapoptotic activity of Vpr.
Figures

Similar articles
-
The C-terminal moiety of HIV-1 Vpr induces cell death via a caspase-independent mitochondrial pathway.Cell Death Differ. 2002 Nov;9(11):1212-9. doi: 10.1038/sj.cdd.4401089. Cell Death Differ. 2002. PMID: 12404120
-
Heat-shock protein 70 can replace viral protein R of HIV-1 during nuclear import of the viral preintegration complex.Exp Cell Res. 2000 Sep 15;259(2):398-403. doi: 10.1006/excr.2000.4992. Exp Cell Res. 2000. PMID: 10964507
-
Suppressive effect of elongation factor 2 on apoptosis induced by HIV-1 viral protein R.Apoptosis. 2006 Mar;11(3):377-88. doi: 10.1007/s10495-006-4030-9. Apoptosis. 2006. PMID: 16520893
-
Human immunodeficiency virus type 1 (HIV-1) Vpr-regulated cell death: insights into mechanism.Cell Death Differ. 2005 Aug;12 Suppl 1:962-70. doi: 10.1038/sj.cdd.4401583. Cell Death Differ. 2005. PMID: 15832179 Review.
-
Interactions of HIV-1 viral protein R with host cell proteins.Adv Pharmacol. 2007;55:233-60. doi: 10.1016/S1054-3589(07)55007-6. Adv Pharmacol. 2007. PMID: 17586317 Review. No abstract available.
Cited by
-
HIV-1 Vpr triggers mitochondrial destruction by impairing Mfn2-mediated ER-mitochondria interaction.PLoS One. 2012;7(3):e33657. doi: 10.1371/journal.pone.0033657. Epub 2012 Mar 16. PLoS One. 2012. PMID: 22438978 Free PMC article.
-
Vpr-host interactions during HIV-1 viral life cycle.J Neuroimmune Pharmacol. 2011 Jun;6(2):216-29. doi: 10.1007/s11481-011-9261-z. Epub 2011 Feb 12. J Neuroimmune Pharmacol. 2011. PMID: 21318276 Free PMC article. Review.
-
The anti-apoptotic protein HAX-1 interacts with SERCA2 and regulates its protein levels to promote cell survival.Mol Biol Cell. 2009 Jan;20(1):306-18. doi: 10.1091/mbc.e08-06-0587. Epub 2008 Oct 29. Mol Biol Cell. 2009. PMID: 18971376 Free PMC article.
-
Analysis of HAX-1 gene expression in esophageal squamous cell carcinoma.Diagn Pathol. 2013 Mar 25;8:47. doi: 10.1186/1746-1596-8-47. Diagn Pathol. 2013. PMID: 23531395 Free PMC article.
-
Deregulation of mitochondrial membrane potential by mitochondrial insertion of granzyme B and direct Hax-1 cleavage.J Biol Chem. 2010 Jul 16;285(29):22461-72. doi: 10.1074/jbc.M109.086587. Epub 2010 Apr 13. J Biol Chem. 2010. PMID: 20388708 Free PMC article.
References
-
- Ayyavoo, V., A. Mahboubi, S. Mahalingam, R. Ramalingam, S. Kudchodkar, W. V. Williams, D. R. Green, and D. B. Weiner. 1997. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor κB. Nat. Med. 3:1117-1123. - PubMed
-
- Belzacq, A. S., H. L. Vieira, F. Verrier, G. Vandecasteele, I. Cohen, M. C. Prevost, E. Larquet, F. Pariselli, P. X. Petit, A. Kahn, R. Rizzuto, C. Brenner, and G. Kroemer. 2003. Bcl-2 and Bax modulate adenine nucleotide translocase activity. Cancer Res. 63:541-546. - PubMed
-
- Boya, P., A. L. Pauleau, D. Poncet, R. A. Gonzalez-Polo, N. Zamzami, and G. Kroemer. 2004. Viral proteins targeting mitochondria: controlling cell death. Biochim. Biophys. Acta 1659:178-189. - PubMed
-
- Brenner, C., H. Cadiou, H. L. Vieira, N. Zamzami, I. Marzo, Z. Xie, B. Leber, D. Andrews, H. Duclohier, J. C. Reed, and G. Kroemer. 2000. Bcl-2 and Bax regulate the channel activity of the mitochondrial adenine nucleotide translocator. Oncogene 19:329-336. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

