The endoplasmic reticulum lumenal domain of the adenovirus type 2 E3-19K protein binds to peptide-filled and peptide-deficient HLA-A*1101 molecules
- PMID: 16227254
- PMCID: PMC1262599
- DOI: 10.1128/JVI.79.21.13317-13325.2005
The endoplasmic reticulum lumenal domain of the adenovirus type 2 E3-19K protein binds to peptide-filled and peptide-deficient HLA-A*1101 molecules
Abstract
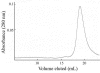
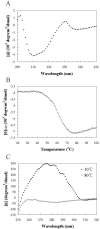
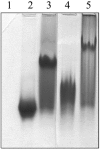
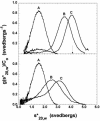
E3-19K is a type I membrane glycoprotein expressed by adenoviruses (Ads) to modulate host antiviral immune responses. We have developed an expression system for the endoplasmic reticulum lumenal domain (residues 1 to 100) of Ad type 2 E3-19K tagged with a C-terminal His6 sequence in baculovirus-infected insect cells. In this system, recombinant E3-19K is secreted into the culture medium. A characterization of soluble E3-19K by analytical ultracentrifugation and circular dichroism showed that the protein is monomeric and adopts a stable and correctly folded tertiary structure. Using a gel mobility shift assay and analytical ultracentrifugation, we showed that soluble E3-19K associates with soluble peptide-filled and peptide-deficient HLA-A*1101 molecules. This is the first example of a viral immunomodulatory protein that interacts with conformationally distinct forms of class I major histocompatibility complex molecules. The E3-19K/HLA-A*1101 complexes formed in a 1:1 stoichiometry with equilibrium dissociation constants (Kd) of 50 +/- 10 nM for peptide-filled molecules and of about 10 microM for peptide-deficient molecules. A temperature-dependent proteolysis study revealed that the association of E3-19K with peptide-deficient HLA-A*1101 molecules stabilizes the binding groove. Importantly, our studies showed that peptide-deficient HLA-A*1101 molecules sequestered by E3-19K are capable of binding antigenic peptides and maturing into peptide-filled molecules. This firmly establishes that E3-19K does not block binding of antigenic peptides. Together, our results suggest that Ads have evolved to exploit the late and early stages of the class I antigen presentation pathway.
Figures


Similar articles
-
Determinants of the endoplasmic reticulum (ER) lumenal-domain of the adenovirus serotype 2 E3-19K protein for association with and ER-retention of major histocompatibility complex class I molecules.Mol Immunol. 2011 Jan;48(4):532-8. doi: 10.1016/j.molimm.2010.10.017. Epub 2010 Nov 20. Mol Immunol. 2011. PMID: 21094528 Free PMC article.
-
Allele- and locus-specific recognition of class I MHC molecules by the immunomodulatory E3-19K protein from adenovirus.J Immunol. 2007 Apr 1;178(7):4567-75. doi: 10.4049/jimmunol.178.7.4567. J Immunol. 2007. PMID: 17372015
-
Adenovirus E3-19K proteins of different serotypes and subgroups have similar, yet distinct, immunomodulatory functions toward major histocompatibility class I molecules.J Biol Chem. 2011 May 20;286(20):17631-9. doi: 10.1074/jbc.M110.212050. Epub 2011 Mar 25. J Biol Chem. 2011. PMID: 21454588 Free PMC article.
-
Immune evasion by adenovirus E3 proteins: exploitation of intracellular trafficking pathways.Curr Top Microbiol Immunol. 2004;273:29-85. doi: 10.1007/978-3-662-05599-1_2. Curr Top Microbiol Immunol. 2004. PMID: 14674598 Review.
-
Tumor necrosis factor alpha increases expression of adenovirus E3 proteins.Immunobiology. 1995 Jul;193(2-4):186-92. doi: 10.1016/s0171-2985(11)80542-5. Immunobiology. 1995. PMID: 8530142 Review.
Cited by
-
MHC class I antigen presentation: learning from viral evasion strategies.Nat Rev Immunol. 2009 Jul;9(7):503-13. doi: 10.1038/nri2575. Nat Rev Immunol. 2009. PMID: 19498380 Review.
-
Structure of the Adenovirus Type 4 (Species E) E3-19K/HLA-A2 Complex Reveals Species-Specific Features in MHC Class I Recognition.J Immunol. 2016 Aug 15;197(4):1399-407. doi: 10.4049/jimmunol.1600541. Epub 2016 Jul 6. J Immunol. 2016. PMID: 27385781 Free PMC article.
-
Adenovirus E3/19K promotes evasion of NK cell recognition by intracellular sequestration of the NKG2D ligands major histocompatibility complex class I chain-related proteins A and B.J Virol. 2008 May;82(9):4585-94. doi: 10.1128/JVI.02251-07. Epub 2008 Feb 20. J Virol. 2008. PMID: 18287244 Free PMC article.
-
Determinants of the endoplasmic reticulum (ER) lumenal-domain of the adenovirus serotype 2 E3-19K protein for association with and ER-retention of major histocompatibility complex class I molecules.Mol Immunol. 2011 Jan;48(4):532-8. doi: 10.1016/j.molimm.2010.10.017. Epub 2010 Nov 20. Mol Immunol. 2011. PMID: 21094528 Free PMC article.
-
The transmembrane domain of the adenovirus E3/19K protein acts as an endoplasmic reticulum retention signal and contributes to intracellular sequestration of major histocompatibility complex class I molecules.J Virol. 2013 Jun;87(11):6104-17. doi: 10.1128/JVI.03391-12. Epub 2013 Mar 20. J Virol. 2013. PMID: 23514889 Free PMC article.
References
-
- Altmann, F., E. Staudacher, I. B. H. Wilson, and L. Marz. 1999. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj. J. 16:109-123. - PubMed
-
- Beier, D. C., J. H. Cox, D. R. Vining, P. Cresswell, and V. H. Engelhard. 1994. Association of human class I MHC alleles with the adenovirus E3/19K protein. J. Immunol. 152:3862-3872. - PubMed
-
- Bennett, E. M., J. R. Bennink, J. W. Yewdell, and F. M. Brodsky. 1999. Cutting edge: adenovirus E19 has two mechanisms for affecting class I MHC expression. J. Immunol. 162:5049-5052. - PubMed
-
- Bouvier, M., and D. C. Wiley. 1994. Importance of peptide amino and carboxyl termini to the stability of MHC class I molecules. Science 265:398-402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

