The N-terminal domain of the human eIF2beta subunit and the CK2 phosphorylation sites are required for its function
- PMID: 16225457
- PMCID: PMC1386020
- DOI: 10.1042/BJ20050605
The N-terminal domain of the human eIF2beta subunit and the CK2 phosphorylation sites are required for its function
Abstract
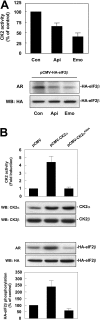
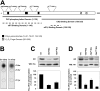
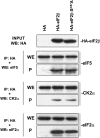
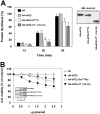
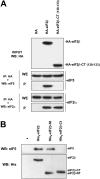
CK2 (protein kinase CK2) is known to phosphorylate eIF2 (eukaryotic translation initiation factor 2) in vitro; however, its implication in this process in living cells has remained to be confirmed. The combined use of chemical inhibitors (emodin and apigenin) of CK2 together with transfection experiments with the wild-type of the K68A kinase-dead mutant form of CK2alpha evidenced the direct involvement of this protein kinase in eIF2beta phosphorylation in cultured HeLa cells. Transfection of HeLa cells with human wild-type eIF2beta or its phosphorylation site mutants showed Ser2 as the main site for constitutive eIF2beta phosphorylation, whereas phosphorylation at Ser67 seems more restricted. In vitro phosphorylation of eIF2beta also pointed to Ser2 as a preferred site for CK2 phosphorylation. Overexpression of the eIF2beta S2/67A mutant slowed down the rate of protein synthesis stimulated by serum, although less markedly than the overexpression of the Delta2-138 N-terminal-truncated form of eIF2beta (eIF2beta-CT). Mutation at Ser2 and Ser67 did not affect eIF2beta integrating into the eIF2 trimer or being able to complex with eIF5 and CK2alpha. The eIF2beta-CT form was also incorporated into the eIF2 trimer but did not bind to eIF5. Overexpression of eIF2beta slightly decreased HeLa cell viability, an effect that was more evident when overexpressing the eIF2beta S2/67A mutant. Cell death was particularly marked when overexpressing the eIF2beta-CT form, being detectable at doses where eIF2beta and eIF2beta S2/67A were ineffective. These results suggest that Ser2 and Ser67 contribute to the important role of the N-terminal region of eIF2beta for its function in mammals.
Figures



Similar articles
-
Eukaryotic translation-initiation factor eIF2beta binds to protein kinase CK2: effects on CK2alpha activity.Biochem J. 2003 Nov 1;375(Pt 3):623-31. doi: 10.1042/BJ20030915. Biochem J. 2003. PMID: 12901717 Free PMC article.
-
Cross talk between protein kinase CK2 and eukaryotic translation initiation factor eIF2beta subunit.Mol Cell Biochem. 2005 Jun;274(1-2):53-61. doi: 10.1007/s11010-005-3081-5. Mol Cell Biochem. 2005. PMID: 16335529
-
The translation initiation factor eIF2beta is an interactor of protein phosphatase-1.Biochem J. 2006 Dec 1;400(2):377-83. doi: 10.1042/BJ20060758. Biochem J. 2006. PMID: 16987104 Free PMC article.
-
The regulatory beta subunit of protein kinase CK2 contributes to the recognition of the substrate consensus sequence. A study with an eIF2 beta-derived peptide.Biochemistry. 2008 Aug 12;47(32):8317-25. doi: 10.1021/bi800216d. Epub 2008 Jul 18. Biochemistry. 2008. PMID: 18636746
-
Conserved sequences in the beta subunit of archaeal and eukaryal translation initiation factor 2 (eIF2), absent from eIF5, mediate interaction with eIF2gamma.Biochem J. 2000 May 1;347 Pt 3(Pt 3):703-9. Biochem J. 2000. PMID: 10769173 Free PMC article.
Cited by
-
Inhibition of casein kinase 2 sensitizes mantle cell lymphoma to venetoclax through MCL-1 downregulation.Haematologica. 2023 Mar 1;108(3):797-810. doi: 10.3324/haematol.2022.281668. Haematologica. 2023. PMID: 36226498 Free PMC article.
-
Cross-species proteomics reveals specific modulation of signaling in cancer and stromal cells by phosphoinositide 3-kinase (PI3K) inhibitors.Mol Cell Proteomics. 2014 Jun;13(6):1457-70. doi: 10.1074/mcp.M113.035204. Epub 2014 Mar 19. Mol Cell Proteomics. 2014. PMID: 24648465 Free PMC article.
-
Phosphorylation of plant translation initiation factors by CK2 enhances the in vitro interaction of multifactor complex components.J Biol Chem. 2009 Jul 31;284(31):20615-28. doi: 10.1074/jbc.M109.007658. Epub 2009 Jun 9. J Biol Chem. 2009. PMID: 19509420 Free PMC article.
-
Signaling Pathways Involved in the Regulation of mRNA Translation.Mol Cell Biol. 2018 May 29;38(12):e00070-18. doi: 10.1128/MCB.00070-18. Print 2018 Jun 15. Mol Cell Biol. 2018. PMID: 29610153 Free PMC article. Review.
-
Phosphoproteomics of colon cancer metastasis: comparative mass spectrometric analysis of the isogenic primary and metastatic cell lines SW480 and SW620.Anal Bioanal Chem. 2017 Mar;409(7):1749-1763. doi: 10.1007/s00216-016-0125-5. Epub 2016 Dec 16. Anal Bioanal Chem. 2017. PMID: 27987026 Free PMC article.
References
-
- Preiss T., Hentze M. W. Starting the protein synthesis: eukaryotic translation initiation. BioEssays. 2003;25:1201–1211. - PubMed
-
- Kapp L. D., Lorsh J. R. The molecular mechanisms of eukaryotic translation. Annu. Rev. Biochem. 2004;73:657–704. - PubMed
-
- Kimball S. R. Eukaryotic initiation factor eIF2. Int. J. Biochem. Cell Biol. 1999;31:25–29. - PubMed
-
- Krishnamoorthy T., Pavitt G. D., Zhang F., Dever T. E., Hinnebusch A. G. Tight binding of the phosphorylated α subunit of initiation factor 2 (eIF2α) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Mol. Cell. Biol. 2001;21:5018–5030. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

