Chemokines: integrators of pain and inflammation
- PMID: 16224455
- PMCID: PMC2792904
- DOI: 10.1038/nrd1852
Chemokines: integrators of pain and inflammation
Abstract
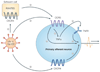
Chronic (neuropathic) pain is one of the most widespread and intractable of human complaints, as well as being one of the most difficult syndromes to treat successfully with drugs or surgery. The development of new therapeutic approaches to the treatment of painful neuropathies requires a better understanding of the mechanisms that underlie the development of these chronic pain syndromes. It is clear that inflammatory responses often accompany the development of neuropathic pain, and here we discuss the idea that chemokines might be key to integrating the development of pain and inflammation and could furnish new leads in the search for effective analgesic agents for the treatment of painful neuropathies.
Figures



Similar articles
-
Painful neuropathies.Curr Opin Neurol. 2003 Oct;16(5):623-8. doi: 10.1097/01.wco.0000093106.34793.06. Curr Opin Neurol. 2003. PMID: 14501847 Review.
-
Force-dependent development of neuropathic central pain and time-related CCL2/CCR2 expression after graded spinal cord contusion injuries of the rat.J Neurotrauma. 2008 May;25(5):427-48. doi: 10.1089/neu.2007.0431. J Neurotrauma. 2008. PMID: 18338959
-
Chemokines in cancer related inflammation.Exp Cell Res. 2011 Mar 10;317(5):664-73. doi: 10.1016/j.yexcr.2010.11.013. Epub 2010 Dec 4. Exp Cell Res. 2011. PMID: 21134366 Review.
-
Endocannabinoids and pain: spinal and peripheral analgesia in inflammation and neuropathy.Prostaglandins Leukot Essent Fatty Acids. 2002 Feb-Mar;66(2-3):243-56. doi: 10.1054/plef.2001.0362. Prostaglandins Leukot Essent Fatty Acids. 2002. PMID: 12052040 Review.
-
[Chemokines and attraction of myeloid cells in peripheral neuropathic pains].Biol Aujourdhui. 2014;208(1):31-44. doi: 10.1051/jbio/20140011. Epub 2014 Jun 23. Biol Aujourdhui. 2014. PMID: 24948017 Review. French.
Cited by
-
Sphingosine 1-phosphate receptor 2 antagonist JTE-013 increases the excitability of sensory neurons independently of the receptor.J Neurophysiol. 2012 Sep;108(5):1473-83. doi: 10.1152/jn.00825.2011. Epub 2012 Jun 6. J Neurophysiol. 2012. PMID: 22673325 Free PMC article.
-
Cellular and subcellular evidence for neuronal interaction between the chemokine stromal cell-derived factor-1/CXCL 12 and vasopressin: regulation in the hypothalamo-neurohypophysial system of the Brattleboro rats.Endocrinology. 2008 Jan;149(1):310-9. doi: 10.1210/en.2007-1097. Epub 2007 Sep 27. Endocrinology. 2008. PMID: 17901225 Free PMC article.
-
Potentiation of morphine antinociception and inhibition of diabetic neuropathic pain by the multi-chemokine receptor antagonist peptide RAP-103.Life Sci. 2022 Oct 1;306:120788. doi: 10.1016/j.lfs.2022.120788. Epub 2022 Jul 9. Life Sci. 2022. PMID: 35817166 Free PMC article.
-
miRNA-23a/CXCR4 regulates neuropathic pain via directly targeting TXNIP/NLRP3 inflammasome axis.J Neuroinflammation. 2018 Jan 31;15(1):29. doi: 10.1186/s12974-018-1073-0. J Neuroinflammation. 2018. PMID: 29386025 Free PMC article.
-
CCR2 upregulation in DRG neurons plays a crucial role in gastric hyperalgesia associated with diabetic gastropathy.Mol Pain. 2018 Jan-Dec;14:1744806917751322. doi: 10.1177/1744806917751322. Mol Pain. 2018. PMID: 29359616 Free PMC article.
References
-
- Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. - PubMed
-
- Lewis W, Day BJ, Copeland WC. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nature Rev. Drug Discov. 2003;2:812–822. - PubMed
-
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nature Rev. Immunol. 2005;5:69–81. - PubMed
-
- Martin TJ, Eisenach JC. Pharmacology of opioid and nonopioid analgesics in chronic pain states. J. Pharmacol. Exp. Ther. 2001;299:811–817. - PubMed
-
- Miljanich GP. Ziconotide: neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 2004;11:3029–3040. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

