Genetic evidence for a mammalian retromer complex containing sorting nexins 1 and 2
- PMID: 16214895
- PMCID: PMC1257690
- DOI: 10.1073/pnas.0409558102
Genetic evidence for a mammalian retromer complex containing sorting nexins 1 and 2
Abstract
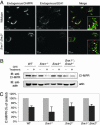
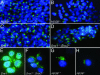
We have previously shown that the putative mammalian retromer components sorting nexins 1 and 2 (Snx1 and Snx2) result in embryonic lethality when simultaneously targeted for deletion in mice, whereas others have shown that Hbeta58 (also known as mVps26), another retromer component, results in similar lethality when targeted for deletion. In the current study, we address the genetic interaction of these mammalian retromer components in mice. Our findings reveal a functional interaction between Hbeta58, SNX1, and SNX2 and strongly suggest that SNX2 plays a more critical role than SNX1 in retromer activity during embryonic development. This genetic evidence supports the existence of mammalian retromer complexes containing SNX1 and SNX2 and identifies SNX2 as an important mediator of retromer biology. Moreover, we find that mammalian retromer complexes containing SNX1 and SNX2 have an essential role in embryonic development that is independent of cation-independent mannose 6-phosphate receptor trafficking.
Figures

Similar articles
-
Genetic analysis of sorting nexins 1 and 2 reveals a redundant and essential function in mice.Mol Biol Cell. 2002 Oct;13(10):3588-600. doi: 10.1091/mbc.e02-03-0145. Mol Biol Cell. 2002. PMID: 12388759 Free PMC article.
-
Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors.Mol Cell Biol. 2007 Feb;27(3):1112-24. doi: 10.1128/MCB.00156-06. Epub 2006 Nov 13. Mol Cell Biol. 2007. PMID: 17101778 Free PMC article.
-
An essential role for SNX1 in lysosomal sorting of protease-activated receptor-1: evidence for retromer-, Hrs-, and Tsg101-independent functions of sorting nexins.Mol Biol Cell. 2006 Mar;17(3):1228-38. doi: 10.1091/mbc.e05-09-0899. Epub 2006 Jan 11. Mol Biol Cell. 2006. PMID: 16407403 Free PMC article.
-
Retromer.Curr Opin Cell Biol. 2008 Aug;20(4):427-36. doi: 10.1016/j.ceb.2008.03.009. Epub 2008 May 9. Curr Opin Cell Biol. 2008. PMID: 18472259 Free PMC article. Review.
-
Canonical and Non-Canonical Roles of SNX1 and SNX2 in Endosomal Membrane Dynamics.Contact (Thousand Oaks). 2023 Nov 28;6:25152564231217867. doi: 10.1177/25152564231217867. eCollection 2023 Jan-Dec. Contact (Thousand Oaks). 2023. PMID: 38033809 Free PMC article. Review.
Cited by
-
Sorting Out Sorting Nexins Functions in the Nervous System in Health and Disease.Mol Neurobiol. 2021 Aug;58(8):4070-4106. doi: 10.1007/s12035-021-02388-9. Epub 2021 May 1. Mol Neurobiol. 2021. PMID: 33931804 Free PMC article. Review.
-
Recycling of autophagosomal components from autolysosomes by the recycler complex.Nat Cell Biol. 2022 Apr;24(4):497-512. doi: 10.1038/s41556-022-00861-8. Epub 2022 Mar 24. Nat Cell Biol. 2022. PMID: 35332264
-
The phosphoinositide kinase PIKfyve/Fab1p regulates terminal lysosome maturation in Caenorhabditis elegans.Mol Biol Cell. 2006 Jul;17(7):3062-74. doi: 10.1091/mbc.e05-12-1120. Mol Biol Cell. 2006. PMID: 16801682 Free PMC article.
-
Evolutionary reconstruction of the retromer complex and its function in Trypanosoma brucei.J Cell Sci. 2011 May 1;124(Pt 9):1496-509. doi: 10.1242/jcs.081596. J Cell Sci. 2011. PMID: 21502137 Free PMC article.
-
Spatiotemporal regulation of the hepatocyte growth factor receptor MET activity by sorting nexins 1/2 in HCT116 colorectal cancer cells.Biosci Rep. 2024 Jun 26;44(6):BSR20240182. doi: 10.1042/BSR20240182. Biosci Rep. 2024. PMID: 38836326 Free PMC article.
References
-
- Carlton, J., Bujny, M., Peter, B. J., Oorschot, V. M., Rutherford, A., Mellor, H., Klumperman, J., McMahon, H. T. & Cullen, P. J. (2004) Curr. Biol. 14, 1791-1800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

