Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis
- PMID: 16212809
- PMCID: PMC1871724
- DOI: 10.1215/S1152851705000050
Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis
Abstract
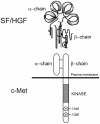
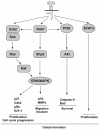
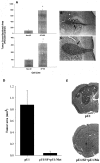
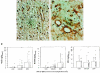
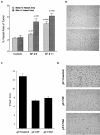
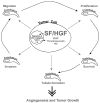
The multifunctional growth factor scatter factor/hepatocyte growth factor (SF/HGF) and its receptor tyrosine kinase c-Met have emerged as key determinants of brain tumor growth and angiogenesis. SF/HGF and c-Met are expressed in brain tumors, the expression levels frequently correlating with tumor grade, tumor blood vessel density, and poor prognosis. Overexpression of SF/HGF and/or c-Met in brain tumor cells enhances their tumorigenicity, tumor growth, and tumor-associated angiogenesis. Conversely, inhibition of SF/HGF and c-Met in experimental tumor xenografts leads to inhibition of tumor growth and tumor angiogenesis. SF/HGF is expressed and secreted mainly by tumor cells and acts on c-Met receptors that are expressed in tumor cells and vascular endothelial cells. Activation of c-Met leads to induction of proliferation, migration, and invasion and to inhibition of apoptosis in tumor cells as well as in tumor vascular endothelial cells. Activation of tumor endothelial c-Met also induces extracellular matrix degradation, tubule formation, and angiogenesis in vivo. SF/HGF induces brain tumor angiogenesis directly through only partly known mechanisms and indirectly by regulating other angiogenic pathways such as VEGF. Different approaches to inhibiting SF/HGF and c-Met have been recently developed. These include receptor antagonism with SF/HGF fragments such as NK4, SF/HGF, and c-Met expression inhibition with U1snRNA/ribozymes; competitive ligand binding with soluble Met receptors; neutralizing antibodies to SF/HGF; and small molecular tyrosine kinase inhibitors. Use of these inhibitors in experimental tumor models leads to inhibition of tumor growth and angiogenesis. In this review, we summarize current knowledge of how the SF/HGF:c-Met pathway contributes to brain tumor malignancy with a focus on glioma angiogenesis.
Figures
Similar articles
-
In vivo targeting of SF/HGF and c-met expression via U1snRNA/ribozymes inhibits glioma growth and angiogenesis and promotes apoptosis.FASEB J. 2002 Jan;16(1):108-10. doi: 10.1096/fj.01-0421fje. Epub 2001 Nov 29. FASEB J. 2002. PMID: 11729097
-
Scatter factor/hepatocyte growth factor (SF/HGF) content and function in human gliomas.Int J Dev Neurosci. 1999 Aug-Oct;17(5-6):517-30. doi: 10.1016/s0736-5748(99)00008-8. Int J Dev Neurosci. 1999. PMID: 10571413 Review.
-
The scatter factor/hepatocyte growth factor: c-met pathway in human embryonal central nervous system tumor malignancy.Cancer Res. 2005 Oct 15;65(20):9355-62. doi: 10.1158/0008-5472.CAN-05-1946. Cancer Res. 2005. PMID: 16230398
-
Expression of hepatocyte growth factor/scatter factor and its receptor c-Met in brain tumors: evidence for a role in progression of astrocytic tumors (Review).Int J Mol Med. 1999 May;3(5):531-6. doi: 10.3892/ijmm.3.5.531. Int J Mol Med. 1999. PMID: 10202187 Review.
-
Reversion of human glioblastoma malignancy by U1 small nuclear RNA/ribozyme targeting of scatter factor/hepatocyte growth factor and c-met expression.J Natl Cancer Inst. 1999 Sep 15;91(18):1548-56. doi: 10.1093/jnci/91.18.1548. J Natl Cancer Inst. 1999. PMID: 10491431
Cited by
-
In vivo detection of c-Met expression in a rat C6 glioma model.J Cell Mol Med. 2008 Jan-Feb;12(1):174-86. doi: 10.1111/j.1582-4934.2008.00220.x. Epub 2007 Jan 9. J Cell Mol Med. 2008. PMID: 18194445 Free PMC article.
-
Fusion Genes Altered in Adult Malignant Gliomas.Front Neurol. 2021 Oct 4;12:715206. doi: 10.3389/fneur.2021.715206. eCollection 2021. Front Neurol. 2021. PMID: 34671307 Free PMC article. Review.
-
H1/pHGFK1 nanoparticles exert anti-tumoural and radiosensitising effects by inhibition of MET in glioblastoma.Br J Cancer. 2018 Feb 20;118(4):522-533. doi: 10.1038/bjc.2017.461. Epub 2018 Jan 18. Br J Cancer. 2018. PMID: 29348487 Free PMC article.
-
High-Content Analysis-Based Sensitivity Prediction and Novel Therapeutics Screening for c-Met-Addicted Glioblastoma.Cancers (Basel). 2021 Jan 20;13(3):372. doi: 10.3390/cancers13030372. Cancers (Basel). 2021. PMID: 33498427 Free PMC article.
-
Interleukin 6 and cancer resistance in glioblastoma multiforme.Neurosurg Rev. 2024 Sep 5;47(1):541. doi: 10.1007/s10143-024-02783-5. Neurosurg Rev. 2024. PMID: 39231832 Review.
References
-
- Abounader R, Ranganathan S, Lal B, Fielding K, Book A, Dietz H, Burger P, Laterra J. Reversion of human glioblastoma malignancy by U1 small nuclear RNA/ribozyme targeting of scatter factor/hepatocyte growth factor and c-met expression. J Natl Cancer Inst. 1999;91:1548–1556. - PubMed
-
- Abounader R, Lal B, Luddy C, Koe G, Davidson B, Rosen EM, Laterra J. In vivo targeting of SF/HGF and c-met expression via U1snRNA/ribozymes inhibits glioma growth and angiogenesis and promotes apoptosis. FASEB J. 2002;16:108–110. - PubMed
-
- Abounader R, Reznik T, Kuchner E, Kwon S, Saldanha U, Dasgupta N, Russel J, Eberhart C, Laterra J. Characterization of the scatter factor/hepatocyte growth factor: c-Met pathway in human medulloblastoma malignancy. Am Assoc Cancer Res. 2004a Available at http://aacr04.agora.com/planner (abstract 4348) - PubMed
-
- Abounader R, Montgomery R, Dietz H, Laterra J. Design and expression of chimeric U1/ribozyme transgenes. Methods Mol Biol. 2004b;252:209–219. - PubMed
-
- Alexandrakis MG, Passam FJ, Ganotakis E, Dafnis E, Dambaki C, Konsolas J, Kyriakou DS, Stathopoulos E. Bone marrow microvascular density and angiogenic growth factors in multiple myeloma. Clin Chem Lab Med. 2004;42:1122–1126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

