Reassembly of active caspase-3 is facilitated by the propeptide
- PMID: 16203739
- PMCID: PMC3119714
- DOI: 10.1074/jbc.M505834200
Reassembly of active caspase-3 is facilitated by the propeptide
Abstract
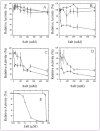
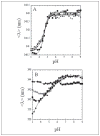
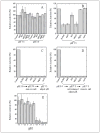
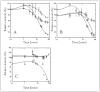
Changes in ionic homeostasis are early events leading up to the commitment to apoptosis. Although the direct effects of cations on caspase-3 activity have been examined, comparable studies on procaspase-3 are lacking. In addition, the effects of salts on caspase structure have not been examined. We have studied the effects of cations on the activities and conformations of caspase-3 and an uncleavable mutant of procaspase-3 that is enzymatically active. The results show that caspase-3 is more sensitive to changes in pH and ion concentrations than is the zymogen. This is due to the loss of both an intact intersubunit linker and the prodomain. The results show that, although the caspase-3 subunits reassemble to the heterotetramer, the activity return is low after the protein is incubated at or below pH 4.5 and then returned to pH 7.5. The data further show that the irreversible step in assembly results from heterotetramer rather than heterodimer dissociation and demonstrate that the active site does not form properly following reassembly. However, active-site formation is fully reversible when reassembly occurs in the presence of the prodomain, and this effect is specific for the propeptide of caspase-3. The data show that the prodomain facilitates both dimerization and active-site formation in addition to stabilizing the native structure. Overall, the results show that the prodomain acts as an intramolecular chaperone during assembly of the (pro)caspase subunits and increases the efficiency of formation of the native conformation.
Figures





Similar articles
-
Ionic interactions near the loop L4 are important for maintaining the active-site environment and the dimer stability of (pro)caspase 3.Biochem J. 2004 Dec 15;384(Pt 3):515-25. doi: 10.1042/BJ20040693. Biochem J. 2004. PMID: 15312047 Free PMC article.
-
The biochemical mechanism of caspase-2 activation.Cell Death Differ. 2004 Nov;11(11):1234-41. doi: 10.1038/sj.cdd.4401492. Cell Death Differ. 2004. PMID: 15297885
-
Human caspase-7 activity and regulation by its N-terminal peptide.J Biol Chem. 2003 Sep 5;278(36):34042-50. doi: 10.1074/jbc.M305110200. Epub 2003 Jun 24. J Biol Chem. 2003. PMID: 12824163
-
Structure and zymogen activation of caspases.Biophys Chem. 2002 Dec 10;101-102:145-53. doi: 10.1016/s0301-4622(02)00151-5. Biophys Chem. 2002. PMID: 12487996 Review.
-
Caspase activation: revisiting the induced proximity model.Cell. 2004 Jun 25;117(7):855-8. doi: 10.1016/j.cell.2004.06.007. Cell. 2004. PMID: 15210107 Review.
Cited by
-
Substrate-induced conformational changes occur in all cleaved forms of caspase-6.J Mol Biol. 2011 Feb 11;406(1):75-91. doi: 10.1016/j.jmb.2010.11.031. Epub 2010 Nov 25. J Mol Biol. 2011. PMID: 21111746 Free PMC article.
-
Molecular Characteristics of Cell Pyroptosis and Its Inhibitors: A Review of Activation, Regulation, and Inhibitors.Int J Mol Sci. 2022 Dec 17;23(24):16115. doi: 10.3390/ijms232416115. Int J Mol Sci. 2022. PMID: 36555757 Free PMC article. Review.
-
Intra-molecular chaperone: the role of the N-terminal in conformational selection and kinetic control.Phys Biol. 2009 Feb 4;6(1):013001. doi: 10.1088/1478-3975/6/1/013001. Phys Biol. 2009. PMID: 19193974 Free PMC article.
-
A bifunctional allosteric site in the dimer interface of procaspase-3.Biophys Chem. 2011 Nov;159(1):100-9. doi: 10.1016/j.bpc.2011.05.013. Epub 2011 May 25. Biophys Chem. 2011. PMID: 21645959 Free PMC article.
-
Modifications to a common phosphorylation network provide individualized control in caspases.J Biol Chem. 2018 Apr 13;293(15):5447-5461. doi: 10.1074/jbc.RA117.000728. Epub 2018 Feb 5. J Biol Chem. 2018. PMID: 29414778 Free PMC article.
References
-
- Earnshaw WC, Martins LM, Kaufmann SH. Annu Rev Biochem. 1999;68:383–424. - PubMed
-
- Tewari M, Quan LT, O’Rouke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM. Cell. 1995;81:801–809. - PubMed
-
- Fearnhead HO, McCurrach ME, O’Neill J, Zhang K, Lowe SW, Lazebnik YA. Genes Dev. 1997;11:1266–1276. - PubMed
-
- Deckwerth TL, Johnson EM., Jr Ann N Y Acad Sci. 1993;679:121–131. - PubMed
-
- Datta R, Banach D, Kojima H, Talanian RV, Alnemri ES, Wong WW, Kufe DW. Blood. 1996;88:1936–1943. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

