A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17
- PMID: 16200068
- PMCID: PMC1618871
- DOI: 10.1038/ni1261
A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17
Abstract
Interleukin 17 (IL-17) has been linked to autoimmune diseases, although its regulation and function have remained unclear. Here we have evaluated in vitro and in vivo the requirements for the differentiation of naive CD4 T cells into effector T helper cells that produce IL-17. This process required the costimulatory molecules CD28 and ICOS but was independent of the cytokines and transcription factors required for T helper type 1 or type 2 differentiation. Furthermore, both IL-4 and interferon-gamma negatively regulated T helper cell production of IL-17 in the effector phase. In vivo, antibody to IL-17 inhibited chemokine expression in the brain during experimental autoimmune encephalomyelitis, whereas overexpression of IL-17 in lung epithelium caused chemokine production and leukocyte infiltration. Thus, IL-17 expression characterizes a unique T helper lineage that regulates tissue inflammation.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Figures


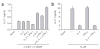
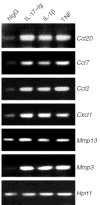


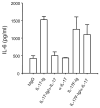
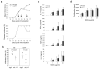
Comment in
-
T(H)-17: a giant step from T(H)1 and T(H)2.Nat Immunol. 2005 Nov;6(11):1069-70. doi: 10.1038/ni1105-1069. Nat Immunol. 2005. PMID: 16239919 No abstract available.
Similar articles
-
The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells.Nat Immunol. 2009 Feb;10(2):167-75. doi: 10.1038/ni.1690. Epub 2008 Dec 21. Nat Immunol. 2009. PMID: 19098919 Free PMC article.
-
A co-stimulatory molecule on activated T cells, H4/ICOS, delivers specific signals in T(h) cells and regulates their responses.Int Immunol. 2002 Jun;14(6):555-66. doi: 10.1093/intimm/dxf022. Int Immunol. 2002. PMID: 12039907
-
Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase.Proc Natl Acad Sci U S A. 2009 Dec 1;106(48):20371-6. doi: 10.1073/pnas.0911573106. Epub 2009 Nov 13. Proc Natl Acad Sci U S A. 2009. PMID: 19915142 Free PMC article.
-
Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells.Nat Rev Immunol. 2006 Apr;6(4):329-33. doi: 10.1038/nri1807. Nat Rev Immunol. 2006. PMID: 16557264 Review.
-
Immune regulation by novel costimulatory molecules.Immunol Res. 2003;28(1):39-48. doi: 10.1385/IR:28:1:39. Immunol Res. 2003. PMID: 12947223 Review.
Cited by
-
Prior Trichinella spiralis infection protects against Schistosoma mansoni induced hepatic fibrosis.Front Vet Sci. 2024 Oct 8;11:1443267. doi: 10.3389/fvets.2024.1443267. eCollection 2024. Front Vet Sci. 2024. PMID: 39439825 Free PMC article.
-
MiR-27a inhibits the growth and metastasis of multiple myeloma through regulating Th17/Treg balance.PLoS One. 2024 Oct 16;19(10):e0311419. doi: 10.1371/journal.pone.0311419. eCollection 2024. PLoS One. 2024. PMID: 39413115 Free PMC article.
-
Elevated serum IL-17 A and CCL20 levels as potential biomarkers in major psychotic disorders: a case-control study.BMC Psychiatry. 2024 Oct 11;24(1):677. doi: 10.1186/s12888-024-06032-3. BMC Psychiatry. 2024. PMID: 39394574 Free PMC article.
-
Ulcerative colitis: molecular insights and intervention therapy.Mol Biomed. 2024 Oct 10;5(1):42. doi: 10.1186/s43556-024-00207-w. Mol Biomed. 2024. PMID: 39384730 Free PMC article. Review.
-
Intralesional gene expression profile of JAK-STAT signaling pathway and associated cytokines in Leishmania tropica-infected patients.Front Immunol. 2024 Sep 19;15:1436029. doi: 10.3389/fimmu.2024.1436029. eCollection 2024. Front Immunol. 2024. PMID: 39364404 Free PMC article.
References
-
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. - PubMed
-
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. - PubMed
-
- Dong C, Nurieva RI. Regulation of immune and autoimmune responses by ICOS. J Autoimmun. 2003;21:255–260. - PubMed
-
- Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. - PubMed
-
- Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
