Apoptosis of hepatocytes caused by Punta Toro virus (Bunyaviridae: Phlebovirus) and its implication for Phlebovirus pathogenesis
- PMID: 16192639
- PMCID: PMC1603669
- DOI: 10.1016/S0002-9440(10)61193-5
Apoptosis of hepatocytes caused by Punta Toro virus (Bunyaviridae: Phlebovirus) and its implication for Phlebovirus pathogenesis
Abstract
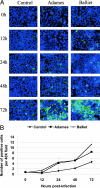
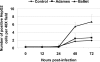
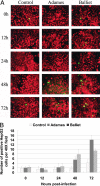
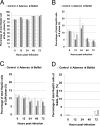
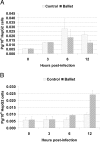
Experimental infection of hamsters with Punta Toro virus (PTV) produces a disease with clinical and pathological similarities to the severe human hemorrhagic fever caused by Rift Valley fever virus (RVFV), thus providing an animal model for RVFV pathogenesis. In this model, hepatocytic apoptosis is the main pathological component of liver injuries that are responsible for severe hemorrhagic manifestations. To further elucidate whether viral replication in hepatocytes directly causes apoptosis, we studied the morphological and biochemical changes of apoptosis in HepG2 cells at different time points after PTV infection. Cellular viability began to decrease 12 hours after infection compared with controls. Caspases 3/7 were activated significantly at 48 and 72 hours after infection, and phosphatidylserine translocation and DNA fragmentation were also detected at 48 and 72 hours. Cell cycle analysis by flow cytometry showed that infected HepG2 cells were arrested at G(0)/G(1) phase. Furthermore, virus titer increased with apoptosis progression, suggesting that viral replication is necessary for the apoptotic process. These results indicate that PTV infection alone, without a secondary inflammatory cellular reaction, induces hepatocytic apoptosis and suggest that future therapeutics for RVFV hemorrhagic disease might target inhibition of cellular apoptotic pathways during the acute infection.
Figures
Similar articles
-
Characterization of cell-death pathways in Punta Toro virus-induced hepatocyte injury.J Gen Virol. 2008 Sep;89(Pt 9):2175-2181. doi: 10.1099/vir.0.2008/001644-0. J Gen Virol. 2008. PMID: 18753227
-
Induction of severe disease in hamsters by two sandfly fever group viruses, Punta toro and Gabek Forest (Phlebovirus, Bunyaviridae), similar to that caused by Rift Valley fever virus.Am J Trop Med Hyg. 2003 Sep;69(3):269-76. Am J Trop Med Hyg. 2003. PMID: 14628943
-
Punta Toro virus (Bunyaviridae, Phlebovirus) infection in mice: strain differences in pathogenesis and host interferon response.Virology. 2009 Dec 5;395(1):143-51. doi: 10.1016/j.virol.2009.09.003. Epub 2009 Sep 26. Virology. 2009. PMID: 19783024 Free PMC article.
-
Severe fever with thrombocytopenia syndrome and its pathogen SFTSV.Microbes Infect. 2015 Feb;17(2):149-54. doi: 10.1016/j.micinf.2014.12.002. Epub 2014 Dec 11. Microbes Infect. 2015. PMID: 25498868 Review.
-
Phleboviruses and the Type I Interferon Response.Viruses. 2016 Jun 22;8(6):174. doi: 10.3390/v8060174. Viruses. 2016. PMID: 27338447 Free PMC article. Review.
Cited by
-
Angiotensin II and dengue.Arch Virol. 2023 Jun 27;168(7):191. doi: 10.1007/s00705-023-05814-6. Arch Virol. 2023. PMID: 37368044 Review.
-
Viral hemorrhagic fevers: current status of endemic disease and strategies for control.Infect Dis Clin North Am. 2006 Jun;20(2):359-93, x. doi: 10.1016/j.idc.2006.02.001. Infect Dis Clin North Am. 2006. PMID: 16762743 Free PMC article. Review. No abstract available.
-
Dengue virus pathogenesis: an integrated view.Clin Microbiol Rev. 2009 Oct;22(4):564-81. doi: 10.1128/CMR.00035-09. Clin Microbiol Rev. 2009. PMID: 19822889 Free PMC article. Review.
-
Crimean-Congo hemorrhagic fever virus-infected hepatocytes induce ER-stress and apoptosis crosstalk.PLoS One. 2012;7(1):e29712. doi: 10.1371/journal.pone.0029712. Epub 2012 Jan 6. PLoS One. 2012. PMID: 22238639 Free PMC article.
-
NSm protein of Rift Valley fever virus suppresses virus-induced apoptosis.J Virol. 2007 Dec;81(24):13335-45. doi: 10.1128/JVI.01238-07. Epub 2007 Oct 3. J Virol. 2007. PMID: 17913816 Free PMC article.
References
-
- Tesh RB. The Genus Phlebovirus and its vectors. Annu Rev Entomol. 1988;33:169–181. - PubMed
-
- Nichol ST. Bunyaviruses. Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Philadelphia: Lippincott Williams & Wilkins,; 2001:1603–1633.
-
- Tetsuro I, Shinji M. Rift Valley fever virus. Uirusu. 2004;54:229–235. - PubMed
-
- Woods CW, Karpati AM, Grein T, McCarthy N, Gaturuku P, Muchiri E, Dunster L, Henderson A, Khan AS, Swanepoel R, Bonmarin I, Martin L, Mann P, Smoak BL, Ryan M, Ksiazek TG, Arthur RR, Ndikuyeze A, Agata NN, Peters CJ, World Health Organization Hemorrhagic Fever Task Force An outbreak of Rift Valley fever in Northeastern Kenya, 1997–98. Emerg Infect Dis. 2002;8:138–144. - PMC - PubMed
-
- Morvan J, Saluzzo JF, Fontenille D, Rollin PE, Coulanges P. Rift Valley fever on the east coast of Madagascar. Res Virol. 1991;142:475–482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

