Functions of cell surface-anchored antigen I/II family and Hsa polypeptides in interactions of Streptococcus gordonii with host receptors
- PMID: 16177339
- PMCID: PMC1230909
- DOI: 10.1128/IAI.73.10.6629-6638.2005
Functions of cell surface-anchored antigen I/II family and Hsa polypeptides in interactions of Streptococcus gordonii with host receptors
Abstract
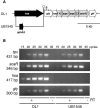
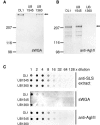
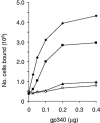
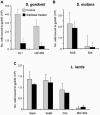
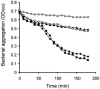
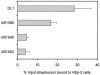
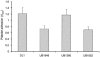
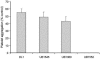
Streptococcus gordonii colonizes multiple sites within the human oral cavity. This colonization depends upon the initial interactions of streptococcal adhesins with host receptors. The adhesins that bind salivary agglutinin glycoprotein (gp340) and human cell surface receptors include the antigen I/II (AgI/II) family polypeptides SspA and SspB and a sialic acid-binding surface protein designated Hsa or GspB. In this study we determined the relative functions of the AgI/II polypeptides and Hsa in interactions of S. gordonii DL1 (Challis) with host receptors. For an isogenic mutant with the sspA and sspB genes deleted the levels of adhesion to surface-immobilized gp340 were reduced 40%, while deletion of the hsa gene alone resulted in >80% inhibition of bacterial cell adhesion to gp340. Adhesion of S. gordonii DL1 cells to gp340 was sialidase sensitive, verifying that Hsa has a major role in mediating sialic acid-specific adhesion to gp340. Conversely, aggregation of S. gordonii cells by fluid-phase gp340 was not affected by deletion of hsa but was eliminated by deletion of the sspA and sspB genes. Deletion of the AgI/II polypeptide genes had no measurable effect on hsa mRNA levels or Hsa surface protein expression, and deletion of hsa did not affect AgI/II polypeptide expression. Further analysis of mutant phenotypes showed that the Hsa and AgI/II proteins mediated adhesion of S. gordonii DL1 to human HEp-2 epithelial cells. Hsa was also a principal streptococcal cell surface component promoting adhesion of human platelets to immobilized streptococci, but Hsa and AgI/II polypeptides acted in concert in mediating streptococcal cell-platelet aggregation. The results suggest that Hsa directs primary adhesion events for S. gordonii DL1 (Challis) with immobilized gp340, epithelial cells, and platelets. AgI/II polypeptides direct gp340-mediated aggregation, facilitate multimodal interactions necessary for platelet aggregation, and modulate S. gordonii-host engagements into biologically productive phenomena.
Figures

Similar articles
-
Differential binding specificities of oral streptococcal antigen I/II family adhesins for human or bacterial ligands.Mol Microbiol. 2005 Mar;55(5):1591-605. doi: 10.1111/j.1365-2958.2005.04495.x. Mol Microbiol. 2005. PMID: 15720563
-
Adherence and internalization of Streptococcus gordonii by epithelial cells involves beta1 integrin recognition by SspA and SspB (antigen I/II family) polypeptides.Cell Microbiol. 2007 Jan;9(1):65-83. doi: 10.1111/j.1462-5822.2006.00768.x. Epub 2006 Jul 31. Cell Microbiol. 2007. PMID: 16879454
-
Fluid- or surface-phase human salivary scavenger protein gp340 exposes different bacterial recognition properties.Infect Immun. 2005 Apr;73(4):2245-52. doi: 10.1128/IAI.73.4.2245-2252.2005. Infect Immun. 2005. PMID: 15784568 Free PMC article.
-
The changing faces of Streptococcus antigen I/II polypeptide family adhesins.Mol Microbiol. 2010 Jul;77(2):276-86. doi: 10.1111/j.1365-2958.2010.07212.x. Epub 2010 May 24. Mol Microbiol. 2010. PMID: 20497507 Free PMC article. Review.
-
[Adhesins of oral streptococci].Nihon Saikingaku Zasshi. 2013;68(2):283-93. doi: 10.3412/jsb.68.283. Nihon Saikingaku Zasshi. 2013. PMID: 23727707 Review. Japanese.
Cited by
-
CcpA regulates biofilm formation and competence in Streptococcus gordonii.Mol Oral Microbiol. 2012 Apr;27(2):83-94. doi: 10.1111/j.2041-1014.2011.00633.x. Epub 2011 Dec 20. Mol Oral Microbiol. 2012. PMID: 22394467 Free PMC article.
-
Serine-Rich Repeat Adhesins Mediate Shear-Enhanced Streptococcal Binding to Platelets.Infect Immun. 2018 May 22;86(6):e00160-18. doi: 10.1128/IAI.00160-18. Print 2018 Jun. Infect Immun. 2018. PMID: 29581195 Free PMC article.
-
Sepsis - it is all about the platelets.Front Immunol. 2023 Jun 7;14:1210219. doi: 10.3389/fimmu.2023.1210219. eCollection 2023. Front Immunol. 2023. PMID: 37350961 Free PMC article. Review.
-
Absence of capsule reveals glycan-mediated binding and recognition of salivary mucin MUC7 by Streptococcus pneumoniae.Mol Oral Microbiol. 2016 Apr;31(2):175-88. doi: 10.1111/omi.12113. Epub 2015 Aug 17. Mol Oral Microbiol. 2016. PMID: 26172471 Free PMC article.
-
Role of D-alanylation of Streptococcus gordonii lipoteichoic acid in innate and adaptive immunity.Infect Immun. 2007 Jun;75(6):3033-42. doi: 10.1128/IAI.01549-06. Epub 2007 Apr 9. Infect Immun. 2007. PMID: 17420241 Free PMC article.
References
-
- Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. - PMC - PubMed
-
- Banas, J. A., and M. M. Vickerman. 2003. Glucan-binding proteins of the oral streptococci. Crit. Rev. Oral Biol. Med. 14:89-99. - PubMed
-
- Barrau, K., A. Boulamery, G. Imbert, J. P. Casalta, G. Habib, T. Messana, J. L. Bonnet, E. Rubinstein, and D. Raoult. 2004. Causative organisms of infective endocarditis according to host status. Clin. Microbiol. Infect. 10:302-308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

