The role of the cysteine-rich domain of Frizzled in Wingless-Armadillo signaling
- PMID: 16163385
- PMCID: PMC1276175
- DOI: 10.1038/sj.emboj.7600817
The role of the cysteine-rich domain of Frizzled in Wingless-Armadillo signaling
Abstract
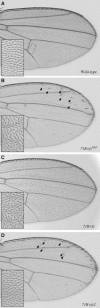
The Frizzled (Fz) receptors contain seven transmembrane helices and an amino-terminal cysteine-rich domain (CRD) that is sufficient and necessary for binding of the ligands, the Wnts. Recent genetic experiments have suggested, however, that the CRD is dispensable for signaling. We engineered fz CRD mutant transgenes and tested them for Wg signaling activity. None of the mutants was functional in cell culture or could fully replace fz in vivo. We also show that replacing the CRD with a structurally distinct Wnt-binding domain, the Wnt inhibitory factor, reconstitutes a functional Wg receptor. We therefore hypothesized that the function of the CRD is to bring Wg in close proximity with the membrane portion of the receptor. We tested this model by substituting Wg itself for the CRD, a manipulation that results in a constitutively active receptor. We propose that Fz activates signaling in two steps: Fz uses its CRD to capture Wg, and once bound Wg interacts with the membrane portion of the receptor to initiate signaling.
Figures


Similar articles
-
Evidence that the cysteine-rich domain of Drosophila Frizzled family receptors is dispensable for transducing Wingless.Proc Natl Acad Sci U S A. 2004 Nov 9;101(45):15961-6. doi: 10.1073/pnas.0407103101. Epub 2004 Oct 28. Proc Natl Acad Sci U S A. 2004. PMID: 15514021 Free PMC article.
-
Genetic evidence that Drosophila frizzled controls planar cell polarity and Armadillo signaling by a common mechanism.Genetics. 2005 Dec;171(4):1643-54. doi: 10.1534/genetics.105.045245. Epub 2005 Aug 5. Genetics. 2005. PMID: 16085697 Free PMC article.
-
Pathway specificity by the bifunctional receptor frizzled is determined by affinity for wingless.Mol Cell. 2000 Jul;6(1):117-26. Mol Cell. 2000. PMID: 10949033
-
Cellular mechanisms of wingless/Wnt signal transduction.Curr Top Dev Biol. 1999;43:153-90. doi: 10.1016/s0070-2153(08)60381-6. Curr Top Dev Biol. 1999. PMID: 9891886 Review.
-
Dishevelled: at the crossroads of divergent intracellular signaling pathways.Mech Dev. 1999 May;83(1-2):27-37. doi: 10.1016/s0925-4773(99)00046-5. Mech Dev. 1999. PMID: 10507837 Review.
Cited by
-
Frizzled2 receives WntA signaling during butterfly wing pattern formation.Development. 2023 Sep 15;150(18):dev201868. doi: 10.1242/dev.201868. Epub 2023 Sep 28. Development. 2023. PMID: 37602496 Free PMC article.
-
The Netrin-related domain of Sfrp1 interacts with Wnt ligands and antagonizes their activity in the anterior neural plate.Neural Dev. 2008 Aug 20;3:19. doi: 10.1186/1749-8104-3-19. Neural Dev. 2008. PMID: 18715500 Free PMC article.
-
Wingless/Wnt signaling in Drosophila: the pattern and the pathway.Mol Reprod Dev. 2013 Nov;80(11):882-94. doi: 10.1002/mrd.22228. Epub 2013 Sep 18. Mol Reprod Dev. 2013. PMID: 24038436 Free PMC article. Review.
-
Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling.Cell. 2008 Jun 13;133(6):1093-105. doi: 10.1016/j.cell.2008.04.048. Cell. 2008. PMID: 18555784 Free PMC article.
-
Asymmetric localizations of LIN-17/Fz and MIG-5/Dsh are involved in the asymmetric B cell division in C. elegans.Dev Biol. 2007 Mar 15;303(2):650-62. doi: 10.1016/j.ydbio.2006.12.002. Epub 2006 Dec 15. Dev Biol. 2007. PMID: 17196955 Free PMC article.
References
-
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, George RA, Lewis SE, Richards S, Ashburner M, Henderson SN, Sutton GG, Wortman JR, Yandell MD, Zhang Q, Chen LX, Brandon RC, Rogers YH, Blazej RG, Champe M, Pfeiffer BD, Wan KH, Doyle C, Baxter EG, Helt G, Nelson CR, Gabor GL, Abril JF, Agbayani A, An HJ, Andrews-Pfannkoch C, Baldwin D, Ballew RM, Basu A, Baxendale J, Bayraktaroglu L, Beasley EM, Beeson KY, Benos PV, Berman BP, Bhandari D, Bolshakov S, Borkova D, Botchan MR, Bouck J, Brokstein P, Brottier P, Burtis KC, Busam DA, Butler H, Cadieu E, Center A, Chandra I, Cherry JM, Cawley S, Dahlke C, Davenport LB, Davies P, de Pablos B, Delcher A, Deng Z, Mays AD, Dew I, Dietz SM, Dodson K, Doup LE, Downes M, Dugan-Rocha S, Dunkov BC, Dunn P, Durbin KJ, Evangelista CC, Ferraz C, Ferriera S, Fleischmann W, Fosler C, Gabrielian AE, Garg NS, Gelbart WM, Glasser K, Glodek A, Gong F, Gorrell JH, Gu Z, Guan P, Harris M, Harris NL, Harvey D, Heiman TJ, Hernandez JR, Houck J, Hostin D, Houston KA, Howland TJ, Wei MH, Ibegwam C, Jalali M, Kalush F, Karpen GH, Ke Z, Kennison JA, Ketchum KA, Kimmel BE, Kodira CD, Kraft C, Kravitz S, Kulp D, Lai Z, Lasko P, Lei Y, Levitsky AA, Li J, Li Z, Liang Y, Lin X, Liu X, Mattei B, McIntosh TC, McLeod MP, McPherson D, Merkulov G, Milshina NV, Mobarry C, Morris J, Moshrefi A, Mount SM, Moy M, Murphy B, Murphy L, Muzny DM, Nelson DL, Nelson DR, Nelson KA, Nixon K, Nusskern DR, Pacleb JM, Palazzolo M, Pittman GS, Pan S, Pollard J, Puri V, Reese MG, Reinert K, Remington K, Saunders RD, Scheeler F, Shen H, Shue BC, Siden-Kiamos I, Simpson M, Skupski MP, Smith T, Spier E, Spradling AC, Stapleton M, Strong R, Sun E, Svirskas R, Tector C, Turner R, Venter E, Wang AH, Wang X, Wang ZY, Wassarman DA, Weinstock GM, Weissenbach J, Williams SM, Woodage T, Worley KC, Wu D, Yang S, Yao QA, Ye J, Yeh RF, Zaveri JS, Zhan M, Zhang G, Zhao Q, Zheng L, Zheng XH, Zhong FN, Zhong W, Zhou X, Zhu S, Zhu X, Smith HO, Gibbs RA, Myers EW, Rubin GM, Venter JC (2000) The genome sequence of Drosophila melanogaster. Science 287: 2185–2195 - PubMed
-
- Alcedo J, Ayzenzon M, Von Ohlen T, Noll M, Hooper JE (1996) The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell 86: 221–232 - PubMed
-
- Bejsovec A (2005) Wnt pathway activation: new relations and locations. Cell 120: 11–14 - PubMed
-
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R (1996) A new member of the Frizzled family from Drosophila functions as a Wingless receptor. Nature 382: 225–230 - PubMed
-
- Bhanot P, Fish M, Jemison JA, Nusse R, Nathans J, Cadigan KM (1999) Frizzled and Dfrizzled-2 function as redundant receptors for Wingless during Drosophila embryonic development. Development 126: 4175–4186 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

