Vectored Gag and Env but not Tat show efficacy against simian-human immunodeficiency virus 89.6P challenge in Mamu-A*01-negative rhesus monkeys
- PMID: 16160159
- PMCID: PMC1211517
- DOI: 10.1128/JVI.79.19.12321-12331.2005
Vectored Gag and Env but not Tat show efficacy against simian-human immunodeficiency virus 89.6P challenge in Mamu-A*01-negative rhesus monkeys
Abstract
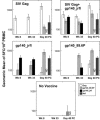
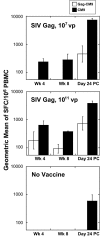
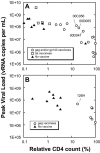
Simian-human immunodeficiency virus (SHIV) challenge studies in rhesus macaques were conducted to evaluate the efficacy of adenovirus-based vaccines in the context of different major histocompatibility complex class I genetic backgrounds and different vaccine compositions. Mamu-A*01 allele-negative rhesus monkeys were immunized with one of the following vaccine constructs: (i) replication-defective recombinant adenovirus type 5 (Ad5) expressing human immunodeficiency virus type 1 (HIV-1) Tat (Ad5/HIVTat); (ii) Ad5 vector expressing simian immunodeficiency virus (SIV) Gag (Ad5/SIVGag); (iii) Ad5 vector expressing the truncated HIV-1(jrfl) Env, gp140 (Ad5/gp140_jrfl); (iv) Ad5 vector expressing the SHIV-89.6P gp140 (Ad5/gp140_89.6P); or (v) the combination of Ad5/SIVGag and Ad5/gp140_jrfl. Following intravenous challenge with SHIV-89.6P, only those cohorts that received vaccines expressing Gag or Env exhibited an attenuation of the acute viremia and associated CD4-cell lymphopenia. While no prechallenge neutralizing antibody titers were detectable in either Ad5/gp140-vaccinated group, an accelerated neutralizing antibody response was observed in the Ad5/gp140_89.6P-vaccinated group upon viral challenge. The set-point viral loads in the Ad5/SIVGag- and Ad5/gp140_jrfl-vaccinated groups were associated with the overall strength of the induced cellular immune responses. To examine the contribution of Mamu-A*01 allele in vaccine efficacy against SHIV-89.6P challenge, Mamu-A*01-positive monkeys were immunized with Ad5/SIVGag. Vaccine-mediated protection was significantly more pronounced in the Mamu-A*01-positive monkeys than in Mamu-A*01-negative monkeys, suggesting the strong contributions of T-cell epitopes restricted by the Mamu-A*01 molecule. The implications of these results in the development of an HIV-1 vaccine will be discussed.
Figures


Similar articles
-
Chimeric adenovirus type 5/35 vector encoding SIV gag and HIV env genes affords protective immunity against the simian/human immunodeficiency virus in monkeys.Virology. 2007 Oct 25;367(2):390-7. doi: 10.1016/j.virol.2007.06.012. Epub 2007 Jul 12. Virology. 2007. PMID: 17628628
-
Highly attenuated rabies virus-based vaccine vectors expressing simian-human immunodeficiency virus89.6P Env and simian immunodeficiency virusmac239 Gag are safe in rhesus macaques and protect from an AIDS-like disease.J Infect Dis. 2007 Apr 1;195(7):980-8. doi: 10.1086/512243. Epub 2007 Feb 20. J Infect Dis. 2007. PMID: 17330788
-
Comparison of vaccine strategies using recombinant env-gag-pol MVA with or without an oligomeric Env protein boost in the SHIV rhesus macaque model.Virology. 2002 Mar 15;294(2):270-81. doi: 10.1006/viro.2001.1345. Virology. 2002. PMID: 12009868
-
Simian immunodeficiency virus-specific cytotoxic T lymphocytes in rhesus monkeys: characterization and vaccine induction.Semin Immunol. 1993 Jun;5(3):215-23. doi: 10.1006/smim.1993.1025. Semin Immunol. 1993. PMID: 8394161 Review.
-
Immune mechanisms associated with protection from vaginal SIV challenge in rhesus monkeys infected with virulence-attenuated SHIV 89.6.J Med Primatol. 2005 Oct;34(5-6):271-81. doi: 10.1111/j.1600-0684.2005.00125.x. J Med Primatol. 2005. PMID: 16128922 Review.
Cited by
-
New directions for HIV vaccine development from animal models.Curr Opin HIV AIDS. 2013 Sep;8(5):376-81. doi: 10.1097/COH.0b013e328363d3a2. Curr Opin HIV AIDS. 2013. PMID: 23836045 Free PMC article. Review.
-
Functional Homology for Antibody-Dependent Phagocytosis Across Humans and Rhesus Macaques.Front Immunol. 2021 May 20;12:678511. doi: 10.3389/fimmu.2021.678511. eCollection 2021. Front Immunol. 2021. PMID: 34093580 Free PMC article.
-
Reservoir cells no longer detectable after a heterologous SHIV challenge with the synthetic HIV-1 Tat Oyi vaccine.Retrovirology. 2006 Jan 27;3:8. doi: 10.1186/1742-4690-3-8. Retrovirology. 2006. PMID: 16441880 Free PMC article.
-
Replicating adenovirus vector prime/protein boost strategies for HIV vaccine development.Expert Opin Biol Ther. 2008 Sep;8(9):1347-63. doi: 10.1517/14712598.8.9.1347. Expert Opin Biol Ther. 2008. PMID: 18694354 Free PMC article. Review.
-
Live attenuated varicella-zoster virus vaccine does not induce HIV target cell activation.J Clin Invest. 2019 Feb 1;129(2):875-886. doi: 10.1172/JCI124473. Epub 2019 Jan 22. J Clin Invest. 2019. PMID: 30511963 Free PMC article. Clinical Trial.
References
-
- Allen, T. M., L. Mortara, B. R. Mothe, M. Liebl, P. Jing, B. Calore, M. Piekarczyk, R. Ruddersdorf, D. H. O'Connor, X. Wang, C. Wang, D. B. Allison, J. D. Altman, A. Sette, R. C. Desrosiers, G. Sutter, and D. I. Watkins. 2002. Tat-vaccinated macaques do not control simian immunodeficiency virus SIVmac239 replication. J. Virol. 76:4108-4112. - PMC - PubMed
-
- Amara, R. R., J. M. Smith, S. I. Staprans, D. C. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, J. M. McNicholl, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J. Virol. 76:6138-6146. - PMC - PubMed
-
- Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. - PubMed
-
- Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233-236. - PubMed
-
- Cafaro, A., A. Caputo, C. Fracasso, M. T. Maggiorella, D. Goletti, S. Baroncelli, M. Pace, L. Sernicola, M. L. Koanga Mogtomo, M. Betti, A. Borsetti, R. Belli, L. Akerblom, F. Corrias, S. Butto, J. Heeney, P. Verani, F. Titti, and B. Ensoli. 1999. Control of SHIV-89.6P-infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat. Med. 5:643-650. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

