Covalently closed circular DNA is the predominant form of duck hepatitis B virus DNA that persists following transient infection
- PMID: 16160150
- PMCID: PMC1211519
- DOI: 10.1128/JVI.79.19.12242-12252.2005
Covalently closed circular DNA is the predominant form of duck hepatitis B virus DNA that persists following transient infection
Abstract
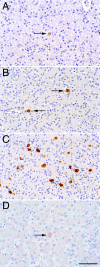
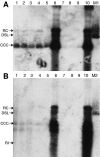
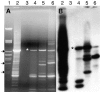
Residual hepatitis B virus (HBV) DNA can be detected in serum and liver after apparent recovery from transient infection. However, it is not known if this residual HBV DNA represents ongoing viral replication and antigen expression. In the current study, ducks inoculated with duck hepatitis B virus (DHBV) were monitored for residual DHBV DNA following recovery from transient infection until 9 months postinoculation (p.i.). Resolution of DHBV infection occurred in 13 out of 15 ducks by 1-month p.i., defined as clearance of DHBV surface antigen-positive hepatocytes from the liver and development of anti-DHBV surface antibodies. At 9 months p.i., residual DHBV DNA was detected using nested PCR in 10/11 liver, 7/11 spleen, 2/11 kidney, 1/11 heart, and 1/11 adrenal samples. Residual DHBV DNA was not detected in serum or peripheral blood mononuclear cells. Within the liver, levels of residual DHBV DNA were 0.0024 to 0.016 copies per cell, 40 to 80% of which were identified as covalently closed circular viral DNA by quantitative PCR assay. This result, which was confirmed by Southern blot hybridization, is consistent with suppressed viral replication or inactive infection. Samples of liver and spleen cells from recovered animals did not transmit DHBV infection when inoculated into 1- to 2-day-old ducklings, and immunosuppressive treatment of ducks with cyclosporine and dexamethasone for 4 weeks did not alter levels of residual DHBV DNA in the liver. These findings further characterize a second form of hepadnavirus persistence in a suppressed or inactive state, quite distinct from the classical chronic carrier state.
Figures

Similar articles
-
The persistence in the liver of residual duck hepatitis B virus covalently closed circular DNA is not dependent upon new viral DNA synthesis.Virology. 2010 Oct 25;406(2):286-92. doi: 10.1016/j.virol.2010.07.013. Epub 2010 Aug 12. Virology. 2010. PMID: 20705309
-
[Inhibitory effect of trisodium phosphonoformate (PFA) on duck hepatitis B virus (DHBV) DNA in vivo].Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 1997 Sep;11(3):277-81. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 1997. PMID: 15617348 Chinese.
-
Antiviral effects of PNA in duck hepatitis B virus infection model.Acta Pharmacol Sin. 2007 Oct;28(10):1652-8. doi: 10.1111/j.1745-7254.2007.00641.x. Acta Pharmacol Sin. 2007. PMID: 17883953
-
[Dynamics of HBV covalently closed circular DNA: amplification and clearance].Zhonghua Gan Zang Bing Za Zhi. 2009 Oct;17(10):794-6. Zhonghua Gan Zang Bing Za Zhi. 2009. PMID: 19874702 Review. Chinese. No abstract available.
-
[Research on the gene structure of duck hepatitis B virus and its encoding proteins].Bing Du Xue Bao. 2012 Nov;28(6):681-8. Bing Du Xue Bao. 2012. PMID: 23367570 Review. Chinese.
Cited by
-
Nucleic acid polymer REP 2139 and nucleos(T)ide analogues act synergistically against chronic hepadnaviral infection in vivo in Pekin ducks.Hepatology. 2018 Jun;67(6):2127-2140. doi: 10.1002/hep.29737. Epub 2018 Feb 23. Hepatology. 2018. PMID: 29251788 Free PMC article.
-
Uracil DNA glycosylase counteracts APOBEC3G-induced hypermutation of hepatitis B viral genomes: excision repair of covalently closed circular DNA.PLoS Pathog. 2013;9(5):e1003361. doi: 10.1371/journal.ppat.1003361. Epub 2013 May 16. PLoS Pathog. 2013. PMID: 23696735 Free PMC article.
-
Activity of nucleic acid polymers in rodent models of HBV infection.Antiviral Res. 2018 Jan;149:26-33. doi: 10.1016/j.antiviral.2017.10.022. Epub 2017 Nov 8. Antiviral Res. 2018. PMID: 29126900 Free PMC article.
-
Immune Tolerant Chronic Hepatitis B: The Unrecognized Risks.Viruses. 2017 Apr 29;9(5):96. doi: 10.3390/v9050096. Viruses. 2017. PMID: 28468285 Free PMC article. Review.
-
Molecular mechanisms underlying occult hepatitis B virus infection.Clin Microbiol Rev. 2012 Jan;25(1):142-63. doi: 10.1128/CMR.00018-11. Clin Microbiol Rev. 2012. PMID: 22232374 Free PMC article. Review.
References
-
- Bertram, E. M., A. R. Jilbert, and I. Kotlarski. 1998. Characterisation of duck thrombocytes. Res. Vet. Sci. 64:267-270. - PubMed
-
- Blum, H. E., T. J. Liang, E. Galun, and J. R. Wands. 1991. Persistence of hepatitis B viral DNA after serological recovery from hepatitis B virus infection. Hepatology 14:56-63. - PubMed
-
- Cacciola, I., T. Pollicino, G. Squadrito, G. Cerenzia, M. E. Orlando, and G. Raimondo. 1999. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N. Engl. J. Med. 341:22-26. - PubMed
-
- Carp, N. Z., J. Saputelli, T. C. Halbherr, W. S. Mason, and A. R. Jilbert. 1991. A technique for liver biopsy performed in Pekin ducks using anesthesia with Telazol. Lab. Anim. Sci. 41:474-475. - PubMed
-
- Delmonico, F. L., and D. R. Snydman. 1998. Organ donor screening for infectious diseases: review of practice and implications for transplantation. Transplantation 65:603-610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

