Anthracyclines, proteasome activity and multi-drug-resistance
- PMID: 16159384
- PMCID: PMC1242219
- DOI: 10.1186/1471-2407-5-114
Anthracyclines, proteasome activity and multi-drug-resistance
Abstract
Background: P-glycoprotein is responsible for the ATP-dependent export of certain structurally unrelated compounds including many chemotherapeutic drugs. Amplification of P-glycoprotein activity can result in multi-drug resistance and is a common cause of chemotherapy treatment failure. Therefore, there is an ongoing search for inhibitors of P-glycoprotein. Observations that cyclosporin A, and certain other substances, inhibit both the proteasome and P-glycoprotein led us to investigate whether anthracyclines, well known substrates of P-gp, also inhibit the function of the proteasome.
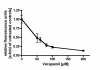
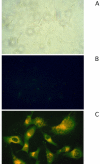
Methods: Proteasome function was measured in cell lysates from ECV304 cells incubated with different doses of verapamil, doxorubicin, daunorubicin, idarubicin, epirubicin, topotecan, mitomycin C, and gemcitabine using a fluorogenic peptide assay. Proteasome function in living cells was monitored using ECV304 cells stably transfected with the gene for an ubiquitin/green fluorescent protein fusion protein. The ability of the proteasome inhibitor MG-132 to affect P-glycoprotein function was monitored by fluorescence due to accumulation of daunorubicin in P-glycoprotein overexpressing KB 8-5 cells.
Results: Verapamil, daunorubicin, doxorubicin, idarubicin, and epirubicin inhibited 26S chymotrypsin-like function in ECV304 extracts in a dose-dependent fashion. With the exception of daunorubicin, 20S proteasome function was also suppressed. The proteasome inhibitor MG-132 caused a dose-dependent accumulation of daunorubicin in KB 8-5 cells that overexpress P-glycoprotein, suggesting that it blocked P-glycoprotein function.
Conclusion: Our data indicate that anthracyclines inhibit the 26S proteasome as well as P-glycoprotein. Use of inhibitors of either pathway in cancer therapy should take this into consideration and perhaps use it to advantage, for example during chemosensitization by proteasome inhibitors.
Figures
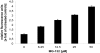
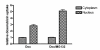
Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD007807. doi: 10.1002/14651858.CD007807.pub4. Cochrane Database Syst Rev. 2020. PMID: 32851663 Free PMC article.
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
Lamotrigine versus levetiracetam or zonisamide for focal epilepsy and valproate versus levetiracetam for generalised and unclassified epilepsy: two SANAD II non-inferiority RCTs.Health Technol Assess. 2021 Dec;25(75):1-134. doi: 10.3310/hta25750. Health Technol Assess. 2021. PMID: 34931602 Clinical Trial.
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2017 Jul 28;7(7):CD007807. doi: 10.1002/14651858.CD007807.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Aug 27;8:CD007807. doi: 10.1002/14651858.CD007807.pub4. PMID: 28752910 Free PMC article. Updated. Review.
Cited by
-
Human Bocavirus Type-1 Capsid Facilitates the Transduction of Ferret Airways by Adeno-Associated Virus Genomes.Hum Gene Ther. 2017 Aug;28(8):612-625. doi: 10.1089/hum.2017.060. Epub 2017 May 10. Hum Gene Ther. 2017. PMID: 28490200 Free PMC article.
-
In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase I.Diabetes. 2009 Aug;58(8):1893-901. doi: 10.2337/db09-0267. Epub 2009 Jun 15. Diabetes. 2009. PMID: 19528375 Free PMC article.
-
Maintenance of endothelial guanosine triphosphate cyclohydrolase I ameliorates diabetic nephropathy.J Am Soc Nephrol. 2013 Jun;24(7):1139-50. doi: 10.1681/ASN.2012080783. Epub 2013 Apr 25. J Am Soc Nephrol. 2013. PMID: 23620395 Free PMC article.
-
Radiation-induced Notch signaling in breast cancer stem cells.Int J Radiat Oncol Biol Phys. 2013 Nov 1;87(3):609-18. doi: 10.1016/j.ijrobp.2013.06.2064. Epub 2013 Aug 27. Int J Radiat Oncol Biol Phys. 2013. PMID: 23992604 Free PMC article.
-
Tyrosine nitration of PA700 links proteasome activation to endothelial dysfunction in mouse models with cardiovascular risk factors.PLoS One. 2012;7(1):e29649. doi: 10.1371/journal.pone.0029649. Epub 2012 Jan 17. PLoS One. 2012. Retraction in: PLoS One. 2023 May 18;18(5):e0286134. doi: 10.1371/journal.pone.0286134. PMID: 22272240 Free PMC article. Retracted.
References
-
- Harding CV, France J, Song R, Farah JM, Chatterjee S, Iqbal M, Siman R. Novel dipeptide aldehydes are proteasome inhibitors and block the MHC-I antigen-processing pathway. J Immunol. 1995;155:1767–1775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

