Facilitation of expression and purification of an antimicrobial peptide by fusion with baculoviral polyhedrin in Escherichia coli
- PMID: 16151084
- PMCID: PMC1214617
- DOI: 10.1128/AEM.71.9.5038-5043.2005
Facilitation of expression and purification of an antimicrobial peptide by fusion with baculoviral polyhedrin in Escherichia coli
Abstract
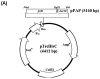
Several fusion strategies have been developed for the expression and purification of small antimicrobial peptides (AMPs) in recombinant bacterial expression systems. However, some of these efforts have been limited by product toxicity to host cells, product proteolysis, low expression levels, poor recovery yields, and sometimes an absence of posttranslational modifications required for biological activity. For the present work, we investigated the use of the baculoviral polyhedrin (Polh) protein as a novel fusion partner for the production of a model AMP (halocidin 18-amino-acid subunit; Hal18) in Escherichia coli. The useful solubility properties of Polh as a fusion partner facilitated the expression of the Polh-Hal18 fusion protein ( approximately 33.6 kDa) by forming insoluble inclusion bodies in E. coli which could easily be purified by inclusion body isolation and affinity purification using the fused hexahistidine tag. The recombinant Hal18 AMP ( approximately 2 kDa) could then be cleaved with hydroxylamine from the fusion protein and easily recovered by simple dialysis and centrifugation. This was facilitated by the fact that Polh was soluble during the alkaline cleavage reaction but became insoluble during dialysis at a neutral pH. Reverse-phase high-performance liquid chromatography was used to further purify the separated recombinant Hal18, giving a final yield of 30% with >90% purity. Importantly, recombinant and synthetic Hal18 peptides showed nearly identical antimicrobial activities against E. coli and Staphylococcus aureus, which were used as representative gram-negative and gram-positive bacteria, respectively. These results demonstrate that baculoviral Polh can provide an efficient and facile platform for the production or functional study of target AMPs.
Figures


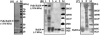

Similar articles
-
Expression and purification of an antimicrobial peptide by fusion with elastin-like polypeptides in Escherichia coli.Appl Biochem Biotechnol. 2010 Apr;160(8):2377-87. doi: 10.1007/s12010-009-8850-2. Epub 2009 Nov 19. Appl Biochem Biotechnol. 2010. PMID: 19924386
-
High and compact formation of baculoviral polyhedrin-induced inclusion body by co-expression of baculoviral FP25 in Escherichia coli.Biotechnol Bioeng. 2007 Apr 15;96(6):1183-90. doi: 10.1002/bit.21193. Biotechnol Bioeng. 2007. PMID: 17004271
-
[Expression and purification of the antimicrobial polypeptide by fusion with elastin-like polypeptide].Fen Zi Xi Bao Sheng Wu Xue Bao. 2008 Jun;41(3):233-7. Fen Zi Xi Bao Sheng Wu Xue Bao. 2008. PMID: 18630603 Chinese.
-
Carrier proteins for fusion expression of antimicrobial peptides in Escherichia coli.Biotechnol Appl Biochem. 2009 Jul 6;54(1):1-9. doi: 10.1042/BA20090087. Biotechnol Appl Biochem. 2009. PMID: 19575694 Free PMC article. Review.
-
Reassessment of inclusion body-based production as a versatile opportunity for difficult-to-express recombinant proteins.Crit Rev Biotechnol. 2018 Aug;38(5):729-744. doi: 10.1080/07388551.2017.1398134. Epub 2017 Nov 10. Crit Rev Biotechnol. 2018. PMID: 29124949 Review.
Cited by
-
High-throughput and facile assay of antimicrobial peptides using pH-controlled fluorescence resonance energy transfer.Antimicrob Agents Chemother. 2006 Oct;50(10):3330-5. doi: 10.1128/AAC.00455-06. Antimicrob Agents Chemother. 2006. PMID: 17005813 Free PMC article.
-
A baculovirus-mediated strategy for full-length plant virus coat protein expression and purification.Virol J. 2013 Aug 15;10:262. doi: 10.1186/1743-422X-10-262. Virol J. 2013. PMID: 23945471 Free PMC article.
-
Strategies for Optimizing the Production of Proteins and Peptides with Multiple Disulfide Bonds.Antibiotics (Basel). 2020 Aug 26;9(9):541. doi: 10.3390/antibiotics9090541. Antibiotics (Basel). 2020. PMID: 32858882 Free PMC article. Review.
-
Investigations into the ability of the peptide, HAL18, to interact with bacterial membranes.Eur Biophys J. 2008 Nov;38(1):37-43. doi: 10.1007/s00249-008-0352-6. Epub 2008 Jul 4. Eur Biophys J. 2008. PMID: 18600320
-
Heterologous Production of Antimicrobial Peptides: Notes to Consider.Protein J. 2024 Apr;43(2):129-158. doi: 10.1007/s10930-023-10174-w. Epub 2024 Jan 5. Protein J. 2024. PMID: 38180586
References
-
- Barrell, P. J., O. W. Liew, and A. J. Conner. 2004. Expressing an antibacterial protein in bacteria for raising antibodies. Protein Expr. Purif. 33:153-159. - PubMed
-
- Boman, H. G. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 13:61-92. - PubMed
-
- Bornstein, P., and G. Balian. 1977. Cleavage at Asn-Gly bonds with hydroxylamine. Methods Enzymol. 47:132-145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

