The cross-species A3 adenosine-receptor antagonist MRS 1292 inhibits adenosine-triggered human nonpigmented ciliary epithelial cell fluid release and reduces mouse intraocular pressure
- PMID: 16146920
- PMCID: PMC3471215
- DOI: 10.1080/02713680590953147
The cross-species A3 adenosine-receptor antagonist MRS 1292 inhibits adenosine-triggered human nonpigmented ciliary epithelial cell fluid release and reduces mouse intraocular pressure
Abstract
Purpose: Antagonists to A3 adenosine receptors (ARs) lower mouse intraocular pressure (IOP), but extension to humans is limited by species variability. We tested whether the specific A3AR antagonist MRS 1292, designed to cross species, mimicks the effects of other A3AR antagonists on cultured human nonpigmented ciliary epithelial (NPE) cells and mouse IOP.
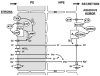
Methods: NPE cell volume was monitored by electronic cell sorting. Mouse IOP was measured with the Servo-Null Micropipette System.
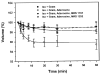
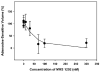
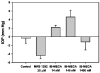
Results: Adenosine triggered A3AR-mediated shrinkage of human NPE cells. Shrinkage was blocked by MRS 1292 (IC50 = 42 +/- 11 nM, p < 0.01) and by another A3AR antagonist effective in this system, MRS 1191. Topical application of the A3AR agonist IB-MECA increased mouse IOP. MRS 1292 reduced IOP by 4.0 +/- 0.8 mmHg at 25-microM droplet concentration (n = 10, p < 0.005).
Conclusions: MRS 1292 inhibits A3AR-mediated shrinkage of human NPE cells and reduces mouse IOP, consistent with its putative action as a cross-species A3 antagonist.
Conflict of interest statement
Disclosure of interest: R.A.S., K.A.J., and M.M.C. are co-inventors on patent applications and issued patents assigned to the University of Pennsylvania and NIH dealing with the use of A3 adenosine-receptor antagonists as agents in treating glaucoma. K.A.J. is a co-inventor of MRS 1292 on a patent assigned to the National Institutes of Health. The other authors have no commercial conflicts of interest.
Figures
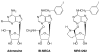
Similar articles
-
Nucleoside-derived antagonists to A3 adenosine receptors lower mouse intraocular pressure and act across species.Exp Eye Res. 2010 Jan;90(1):146-54. doi: 10.1016/j.exer.2009.10.001. Epub 2009 Oct 28. Exp Eye Res. 2010. PMID: 19878673 Free PMC article.
-
Knockout of A3 adenosine receptors reduces mouse intraocular pressure.Invest Ophthalmol Vis Sci. 2002 Sep;43(9):3021-6. Invest Ophthalmol Vis Sci. 2002. PMID: 12202525
-
A3 adenosine receptors regulate Cl- channels of nonpigmented ciliary epithelial cells.Am J Physiol. 1999 Mar;276(3):C659-66. doi: 10.1152/ajpcell.1999.276.3.C659. Am J Physiol. 1999. PMID: 10069993
-
The ins and outs of aqueous humour secretion.Exp Eye Res. 2004 Mar;78(3):625-31. doi: 10.1016/j.exer.2003.09.021. Exp Eye Res. 2004. PMID: 15106942 Review.
-
Swelling-activated chloride channels in aqueous humour formation: on the one side and the other.Acta Physiol (Oxf). 2006 May-Jun;187(1-2):345-52. doi: 10.1111/j.1748-1716.2006.01548.x. Acta Physiol (Oxf). 2006. PMID: 16734771 Review.
Cited by
-
Medicinal chemistry of the A3 adenosine receptor: agonists, antagonists, and receptor engineering.Handb Exp Pharmacol. 2009;(193):123-59. doi: 10.1007/978-3-540-89615-9_5. Handb Exp Pharmacol. 2009. PMID: 19639281 Free PMC article. Review.
-
Nucleoside-derived antagonists to A3 adenosine receptors lower mouse intraocular pressure and act across species.Exp Eye Res. 2010 Jan;90(1):146-54. doi: 10.1016/j.exer.2009.10.001. Epub 2009 Oct 28. Exp Eye Res. 2010. PMID: 19878673 Free PMC article.
-
Recent developments in adenosine receptor ligands and their potential as novel drugs.Biochim Biophys Acta. 2011 May;1808(5):1290-308. doi: 10.1016/j.bbamem.2010.12.017. Epub 2010 Dec 23. Biochim Biophys Acta. 2011. PMID: 21185259 Free PMC article. Review.
-
Introduction to adenosine receptors as therapeutic targets.Handb Exp Pharmacol. 2009;(193):1-24. doi: 10.1007/978-3-540-89615-9_1. Handb Exp Pharmacol. 2009. PMID: 19639277 Free PMC article. Review.
-
John Daly Lecture: Structure-guided Drug Design for Adenosine and P2Y Receptors.Comput Struct Biotechnol J. 2014 Oct 16;13:286-98. doi: 10.1016/j.csbj.2014.10.004. eCollection 2015. Comput Struct Biotechnol J. 2014. PMID: 25973142 Free PMC article. Review.
References
-
- Brubaker RF. Clinical measurement of aqueous dynamics: Implications for addressing glaucoma. In: Civan MM, editor. Eye’s Aqueous Humor: From Secretion to Glaucoma. San Diego: Academic Press; 1998. pp. 234–284.
-
- Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126:498–505. - PubMed
-
- Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–497. - PubMed
-
- The AGIS investigators. The advanced glaucoma intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. - PubMed
-
- Do C-W, Civan MM. Basis of chloride transport in ciliary epithelium. J Membr Biol. 2004;200:1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
