Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation
- PMID: 16140789
- PMCID: PMC1212638
- DOI: 10.1128/JVI.79.18.12112-12116.2005
Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation
Abstract
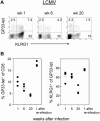
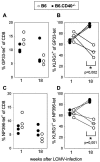
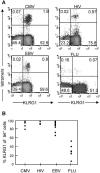
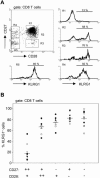
The killer cell lectin-like receptor G1 (KLRG1) is a natural killer cell receptor expressed by T cells that exhibit impaired proliferative capacity. Here, we determined the KLRG1 expression by virus-specific T cells. We found that repetitive and persistent antigen stimulation leads to an increase in KLRG1 expression of virus-specific CD8+ T cells in mice and that virus-specific CD8+ T cells are mostly KLRG1+ in chronic human viral infections (human immunodeficiency virus, cytomegalovirus, and Epstein-Barr virus) but not in resolved infection (influenza virus). Thus, by using KLRG1 as a T-cell marker, our results suggest that the differentiation status and function of virus-specific CD8+ T cells are directly influenced by persistent antigen stimulation.
Figures

Similar articles
-
Analysis of CD127 and KLRG1 expression on hepatitis C virus-specific CD8+ T cells reveals the existence of different memory T-cell subsets in the peripheral blood and liver.J Virol. 2007 Jan;81(2):945-53. doi: 10.1128/JVI.01354-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079288 Free PMC article.
-
The NK receptor KLRG1 is dispensable for virus-induced NK and CD8+ T-cell differentiation and function in vivo.Eur J Immunol. 2010 May;40(5):1303-14. doi: 10.1002/eji.200939771. Eur J Immunol. 2010. PMID: 20201037
-
Persistent expression of CD94/NKG2 receptors by virus-specific CD8 T cells is initiated by TCR-mediated signals.Int Immunol. 2004 Sep;16(9):1333-41. doi: 10.1093/intimm/dxh136. Epub 2004 Aug 9. Int Immunol. 2004. PMID: 15302848
-
Functional plasticity and robustness are essential characteristics of biological systems: lessons learned from KLRG1-deficient mice.Eur J Immunol. 2010 May;40(5):1241-3. doi: 10.1002/eji.201040506. Eur J Immunol. 2010. PMID: 20373518 Review.
-
CD8 T cell dysfunction during chronic viral infection.Curr Opin Immunol. 2007 Aug;19(4):408-15. doi: 10.1016/j.coi.2007.06.004. Epub 2007 Jul 25. Curr Opin Immunol. 2007. PMID: 17656078 Review.
Cited by
-
Molecular signatures of T-cell inhibition in HIV-1 infection.Retrovirology. 2013 Mar 20;10:31. doi: 10.1186/1742-4690-10-31. Retrovirology. 2013. PMID: 23514593 Free PMC article. Review.
-
Combined mTOR inhibition and OX40 agonism enhances CD8(+) T cell memory and protective immunity produced by recombinant adenovirus vaccines.Mol Ther. 2012 Apr;20(4):860-9. doi: 10.1038/mt.2011.281. Epub 2011 Dec 20. Mol Ther. 2012. PMID: 22186790 Free PMC article.
-
KLRG1 impairs CD4+ T cell responses via p16ink4a and p27kip1 pathways: role in hepatitis B vaccine failure in individuals with hepatitis C virus infection.J Immunol. 2014 Jan 15;192(2):649-57. doi: 10.4049/jimmunol.1302069. Epub 2013 Dec 13. J Immunol. 2014. PMID: 24337749 Free PMC article.
-
Buffered memory: a hypothesis for the maintenance of functional, virus-specific CD8(+) T cells during cytomegalovirus infection.Immunol Res. 2011 Dec;51(2-3):195-204. doi: 10.1007/s12026-011-8251-9. Immunol Res. 2011. PMID: 22058020 Review.
-
T Cell Repertoire Maturation Induced by Persistent and Latent Viral Infection Is Insufficient to Induce Costimulation Blockade Resistant Organ Allograft Rejection in Mice.Front Immunol. 2018 Jun 15;9:1371. doi: 10.3389/fimmu.2018.01371. eCollection 2018. Front Immunol. 2018. PMID: 29963060 Free PMC article.
References
-
- Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. - PubMed
-
- Anfossi, N., S. H. Robbins, S. Ugolini, P. Georgel, K. Hoebe, C. Bouneaud, C. Ronet, A. Kaser, C. B. DiCioccio, E. Tomasello, R. S. Blumberg, B. Beutler, S. L. Reiner, L. Alexopoulou, O. Lantz, D. H. Raulet, L. Brossay, and E. Vivier. 2004. Expansion and function of CD8+ T cells expressing Ly49 inhibitory receptors specific for MHC class I molecules. J. Immunol. 173:3773-3782. - PubMed
-
- Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. - PubMed
-
- Azuma, M., J. H. Phillips, and L. L. Lanier. 1993. CD28− T lymphocytes. Antigenic and functional properties. J. Immunol. 150:1147-1159. - PubMed
-
- Bachmann, M. F., L. Hunziker, R. M. Zinkernagel, T. Storni, and M. Kopf. 2004. Maintenance of memory CTL responses by T helper cells and CD40-CD40 ligand: antibodies provide the key. Eur. J. Immunol. 34:317-326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

