High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation
- PMID: 16140584
- PMCID: PMC1473967
- DOI: 10.1016/j.ymthe.2005.07.688
High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation
Abstract
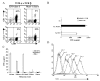
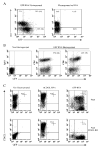
The use of nonviral gene transfer methods in primary lymphocytes has been hampered by low gene transfer efficiency and high transfection-related toxicity. In this report, high gene transfection efficiency with low transfection-related toxicity was achieved by electroporation using in vitro-transcribed mRNA. Using these methods, >90% transgene expression with >80% viable cells was observed in stimulated primary human and murine T lymphocytes transfected with GFP or mCD62L. Electroporation of unstimulated human PBMCs or murine splenocytes with GFP RNA yielded 95 and 56% GFP+ cells, respectively. Electroporation of mRNA for NY-ESO-1, MART-1, and p53 antigen-specific TCRs into human T lymphocytes redirected these lymphocytes to recognize melanoma cell lines in an MHC-restricted manner. The onset of gene expression was rapid (within 30 min) and durable (up to 7 days postelectroporation) using both GFP and TCR-mediated recognition of target cells. There was no adverse effect observed on the T lymphocytes subjected to RNA electroporation evaluated by cell growth rate, annexin-V staining of apoptotic cells, BrdU incorporation, tumor antigen-specific recognition or antigen-specific TCR affinity. The results of this study indicate that mRNA electroporation provides a powerful tool to introduce genes into both human and murine primary T lymphocytes.
Figures


Similar articles
-
Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines.J Immunol. 2005 Apr 1;174(7):4415-23. doi: 10.4049/jimmunol.174.7.4415. J Immunol. 2005. PMID: 15778407 Free PMC article.
-
RNA-transfection of γ/δ T cells with a chimeric antigen receptor or an α/β T-cell receptor: a safer alternative to genetically engineered α/β T cells for the immunotherapy of melanoma.BMC Cancer. 2017 Aug 17;17(1):551. doi: 10.1186/s12885-017-3539-3. BMC Cancer. 2017. PMID: 28818060 Free PMC article.
-
High transfection efficiency of porcine peripheral blood T cells via nucleofection.Vet Immunol Immunopathol. 2011 Dec 15;144(3-4):179-86. doi: 10.1016/j.vetimm.2011.10.003. Epub 2011 Oct 15. Vet Immunol Immunopathol. 2011. PMID: 22055481
-
Activation of tumor-specific T cells by dendritic cells expressing the NY-ESO-1 antigen after transfection with the cationic lipophosphoramide KLN5.J Gene Med. 2008 Jun;10(6):628-36. doi: 10.1002/jgm.1188. J Gene Med. 2008. PMID: 18338820
-
The Ins and Outs of Messenger RNA Electroporation for Physical Gene Delivery in Immune Cell-Based Therapy.Pharmaceutics. 2021 Mar 16;13(3):396. doi: 10.3390/pharmaceutics13030396. Pharmaceutics. 2021. PMID: 33809779 Free PMC article. Review.
Cited by
-
Delivery of RNAi Therapeutics to the Airways-From Bench to Bedside.Molecules. 2016 Sep 20;21(9):1249. doi: 10.3390/molecules21091249. Molecules. 2016. PMID: 27657028 Free PMC article. Review.
-
Regimen-specific effects of RNA-modified chimeric antigen receptor T cells in mice with advanced leukemia.Hum Gene Ther. 2013 Aug;24(8):717-27. doi: 10.1089/hum.2013.075. Hum Gene Ther. 2013. PMID: 23883116 Free PMC article.
-
Synthetic TILs: Engineered Tumor-Infiltrating Lymphocytes With Improved Therapeutic Potential.Front Oncol. 2021 Feb 16;10:593848. doi: 10.3389/fonc.2020.593848. eCollection 2020. Front Oncol. 2021. PMID: 33680923 Free PMC article. Review.
-
Intracellular Delivery of mRNA in Adherent and Suspension Cells by Vapor Nanobubble Photoporation.Nanomicro Lett. 2020 Sep 27;12(1):185. doi: 10.1007/s40820-020-00523-0. Nanomicro Lett. 2020. PMID: 34138203 Free PMC article.
-
Generation of CD8(+) T cells expressing two additional T-cell receptors (TETARs) for personalised melanoma therapy.Cancer Biol Ther. 2015;16(9):1323-31. doi: 10.1080/15384047.2015.1070981. Epub 2015 Jul 15. Cancer Biol Ther. 2015. PMID: 26178065 Free PMC article.
References
-
- Rudoll T, et al. High-efficiency retroviral vector mediated gene transfer into human peripheral blood CD4+ T lymphocytes. Gene Ther. 1996;3:695. - PubMed
-
- Costello E, Munoz M, Buetti E, Meylan PR, Diggelmann H, Thali M. Gene transfer into stimulated and unstimulated T lymphocytes by HIV-1-derived lentiviral vectors. Gene Ther. 2000;7:596. - PubMed
-
- Ferber D. Gene therapy: safer and virus-free? Science. 2001;294:1638. - PubMed
-
- Bell MP, Huntoon CJ, Graham D, McKean DJ. The analysis of costimulatory receptor signaling cascades in normal T lymphocytes using in vitro gene transfer and reporter gene analysis. Nat Med. 2001;7:1155. - PubMed
-
- Van Tendeloo VF, et al. High-level transgene expression in primary human T lymphocytes and adult bone marrow CD34+ cells via electroporation-mediated gene delivery. Gene Ther. 2000;7:1431. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

