Chicken retinas contain a retinoid isomerase activity that catalyzes the direct conversion of all-trans-retinol to 11-cis-retinol
- PMID: 16128572
- PMCID: PMC2851629
- DOI: 10.1021/bi050942m
Chicken retinas contain a retinoid isomerase activity that catalyzes the direct conversion of all-trans-retinol to 11-cis-retinol
Abstract
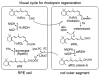
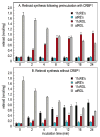
Vertebrate retinas contain two types of light-detecting cells. Rods subserve vision in dim light, while cones provide color vision in bright light. Both contain light-sensitive proteins called opsins. The light-absorbing chromophore in most opsins is 11-cis-retinaldehyde, which is isomerized to all-trans-retinaldehyde by absorption of a photon. Restoration of light sensitivity requires chemical re-isomerization of retinaldehyde by an enzymatic pathway called the visual cycle in the retinal pigment epithelium. The isomerase in this pathway uses all-trans-retinyl esters synthesized by lecithin retinol acyl transferase (LRAT) as the substrate. Several lines of evidence suggest that cone opsins regenerate by a different mechanism. Here we demonstrate the existence of two catalytic activities in chicken retinas. The first is an isomerase activity that effects interconversion of all-trans-retinol and 11-cis-retinol. The second is an ester synthase that effects palmitoyl coenzyme A-dependent synthesis of all-trans- and 11-cis-retinyl esters. Kinetic analysis of these two activities suggests that they act in concert to drive the formation of 11-cis-retinoids in chicken retinas. These activities may be part of a new visual cycle for the regeneration of chromophores in cones.
Figures

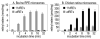




Similar articles
-
Analysis of the retinoid isomerase activities in the retinal pigment epithelium and retina.Methods Mol Biol. 2010;652:329-39. doi: 10.1007/978-1-60327-325-1_19. Methods Mol Biol. 2010. PMID: 20552438 Free PMC article.
-
Acyl CoA:retinol acyltransferase (ARAT) activity is present in bovine retinal pigment epithelium.Exp Eye Res. 2006 Jan;82(1):111-21. doi: 10.1016/j.exer.2005.05.010. Epub 2005 Jul 27. Exp Eye Res. 2006. PMID: 16054134
-
Retinyl esters are the substrate for isomerohydrolase.Biochemistry. 2003 Feb 25;42(7):2229-38. doi: 10.1021/bi026911y. Biochemistry. 2003. PMID: 12590612
-
Vitamin A and Vision.Subcell Biochem. 2016;81:231-259. doi: 10.1007/978-94-024-0945-1_9. Subcell Biochem. 2016. PMID: 27830507 Review.
-
The visual cycle of the cone photoreceptors of the retina.Nutr Rev. 2004 Jul;62(7 Pt 1):283-6. doi: 10.1111/j.1753-4887.2004.tb00053.x. Nutr Rev. 2004. PMID: 15384919 Review.
Cited by
-
Chemistry of the retinoid (visual) cycle.Chem Rev. 2014 Jan 8;114(1):194-232. doi: 10.1021/cr400107q. Epub 2013 Jul 11. Chem Rev. 2014. PMID: 23905688 Free PMC article. Review. No abstract available.
-
iPSC-Derived Retina Transplants Improve Vision in rd1 End-Stage Retinal-Degeneration Mice.Stem Cell Reports. 2017 Jan 10;8(1):69-83. doi: 10.1016/j.stemcr.2016.12.008. Stem Cell Reports. 2017. PMID: 28076757 Free PMC article.
-
Normal cone function requires the interphotoreceptor retinoid binding protein.J Neurosci. 2009 Apr 8;29(14):4616-21. doi: 10.1523/JNEUROSCI.0063-09.2009. J Neurosci. 2009. PMID: 19357286 Free PMC article.
-
The retinal pigment epithelium in health and disease.Curr Mol Med. 2010 Dec;10(9):802-23. doi: 10.2174/156652410793937813. Curr Mol Med. 2010. PMID: 21091424 Free PMC article. Review.
-
RPE65 from cone-dominant chicken is a more efficient isomerohydrolase compared with that from rod-dominant species.J Biol Chem. 2008 Mar 28;283(13):8110-7. doi: 10.1074/jbc.M703654200. Epub 2008 Jan 23. J Biol Chem. 2008. PMID: 18216020 Free PMC article.
References
-
- Arshavsky VY, Lamb TD, Pugh EN. G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–87. - PubMed
-
- Moiseyev G, Crouch RK, Goletz P, Oatis J, Jr, Redmond TM, Ma JX. Retinyl esters are the substrate for isomerohydrolase. Biochemistry. 2003;42:2229–38. - PubMed
-
- Gollapalli DR, Rando RR. All-trans-retinyl esters are the substrates for isomerization in the vertebrate visual cycle. Biochemistry. 2003;42:5809–18. - PubMed
-
- Mata NL, Moghrabi WN, Lee JS, Bui TV, Radu RA, Horwitz J, Travis GH. Rpe65 is a retinyl ester binding protein that presents insoluble substrate to the isomerase in retinal pigment epithelial cells. J Biol Chem. 2004;279:635–43. - PubMed
-
- MacDonald PN, Ong DE. Evidence for a lecithin-retinol acyltransferase activity in the rat small intestine. J Biol Chem. 1988;263:12478–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

