PTP-1B is an essential positive regulator of platelet integrin signaling
- PMID: 16115959
- PMCID: PMC2171339
- DOI: 10.1083/jcb.200503125
PTP-1B is an essential positive regulator of platelet integrin signaling
Abstract
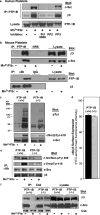
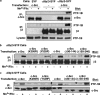
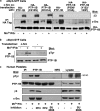
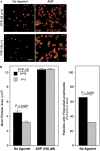
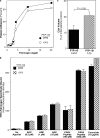
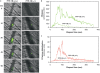
Outside-in integrin alphaIIbbeta3 signaling is required for normal platelet thrombus formation and is triggered by c-Src activation through an unknown mechanism. In this study, we demonstrate an essential role for protein-tyrosine phosphatase (PTP)-1B in this process. In resting platelets, c-Src forms a complex with alphaIIbbeta3 and Csk, which phosphorylates c-Src tyrosine 529 to maintain c-Src autoinhibition. Fibrinogen binding to alphaIIbbeta3 triggers PTP-1B recruitment to the alphaIIbbeta3-c-Src-Csk complex in a manner that is dependent on c-Src and specific tyrosine (tyrosine 152 and 153) and proline (proline 309 and 310) residues in PTP-1B. Studies of PTP-1B-deficient mouse platelets indicate that PTP-1B is required for fibrinogen-dependent Csk dissociation from alphaIIbbeta3, dephosphorylation of c-Src tyrosine 529, and c-Src activation. Furthermore, PTP-1B-deficient platelets are defective in outside-in alphaIIbbeta3 signaling in vitro as manifested by poor spreading on fibrinogen and decreased clot retraction, and they exhibit ineffective Ca2+ signaling and thrombus formation in vivo. Thus, PTP-1B is an essential positive regulator of the initiation of outside-in alphaIIbbeta3 signaling in platelets.
Figures
Similar articles
-
Junctional adhesion molecule-A suppresses platelet integrin αIIbβ3 signaling by recruiting Csk to the integrin-c-Src complex.Blood. 2014 Feb 27;123(9):1393-402. doi: 10.1182/blood-2013-04-496232. Epub 2013 Dec 3. Blood. 2014. PMID: 24300854 Free PMC article.
-
Coordinate interactions of Csk, Src, and Syk kinases with [alpha]IIb[beta]3 initiate integrin signaling to the cytoskeleton.J Cell Biol. 2002 Apr 15;157(2):265-75. doi: 10.1083/jcb.200112113. Epub 2002 Apr 8. J Cell Biol. 2002. PMID: 11940607 Free PMC article.
-
Cross-talk between serine/threonine protein phosphatase 2A and protein tyrosine phosphatase 1B regulates Src activation and adhesion of integrin αIIbβ3 to fibrinogen.J Biol Chem. 2010 Sep 17;285(38):29059-68. doi: 10.1074/jbc.M109.085167. Epub 2010 Jul 8. J Biol Chem. 2010. PMID: 20615878 Free PMC article.
-
Beta3 tyrosine phosphorylation in alphaIIbbeta3 (platelet membrane GP IIb-IIIa) outside-in integrin signaling.Thromb Haemost. 2001 Jul;86(1):246-58. Thromb Haemost. 2001. PMID: 11487013 Review.
-
Integrin αIIbβ3 outside-in signaling.Blood. 2017 Oct 5;130(14):1607-1619. doi: 10.1182/blood-2017-03-773614. Epub 2017 Aug 9. Blood. 2017. PMID: 28794070 Free PMC article. Review.
Cited by
-
A molecular switch that controls cell spreading and retraction.J Cell Biol. 2007 Nov 5;179(3):553-65. doi: 10.1083/jcb.200703185. Epub 2007 Oct 29. J Cell Biol. 2007. PMID: 17967945 Free PMC article.
-
G(12/13) signaling pathways substitute for integrin αIIbβ3-signaling for thromboxane generation in platelets.PLoS One. 2011 Feb 10;6(2):e16586. doi: 10.1371/journal.pone.0016586. PLoS One. 2011. PMID: 21347357 Free PMC article.
-
Double knockouts reveal that protein tyrosine phosphatase 1B is a physiological target of calpain-1 in platelets.Mol Cell Biol. 2007 Sep;27(17):6038-52. doi: 10.1128/MCB.00522-07. Epub 2007 Jun 18. Mol Cell Biol. 2007. PMID: 17576811 Free PMC article.
-
Perspective: Tyrosine phosphatases as novel targets for antiplatelet therapy.Bioorg Med Chem. 2015 Jun 15;23(12):2786-97. doi: 10.1016/j.bmc.2015.03.075. Epub 2015 Apr 4. Bioorg Med Chem. 2015. PMID: 25921264 Free PMC article. Review.
-
Leptin receptor (Lepr) is a negative modulator of bone mechanosensitivity and genetic variations in Lepr may contribute to the differential osteogenic response to mechanical stimulation in the C57BL/6J and C3H/HeJ pair of mouse strains.J Biol Chem. 2010 Nov 26;285(48):37607-18. doi: 10.1074/jbc.M110.169714. Epub 2010 Sep 17. J Biol Chem. 2010. PMID: 20851886 Free PMC article.
References
-
- Brunton, V.G., I.R.J. MacPherson, and M.C. Frame. 2004. Cell adhesion receptors, tyrosine kinases and actin modulators: a complex three-way circuitry. Biochim. Biophys. Acta. 1692:121–144. - PubMed
-
- Buensuceso, C., M. De Virgilio, and S.J. Shattil. 2003. Detection of integrin αIIbβ3 clustering in living cells. J. Biol. Chem. 278:15217–15224. - PubMed
-
- Byzova, T.V., R. Rabbani, S. D'Souza, and E.F. Plow. 1998. Role of integrin αVβ3 in vascular biology. Thromb. Haemost. 80:726–734. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

