TWEAK induces liver progenitor cell proliferation
- PMID: 16110324
- PMCID: PMC1187931
- DOI: 10.1172/JCI23486
TWEAK induces liver progenitor cell proliferation
Erratum in
- J Clin Invest. 2005 Oct;115(10):2955. Baestcher, Manfred [corrected to Baetscher, Manfred]
Abstract
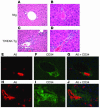
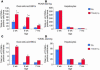
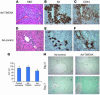
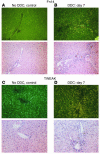
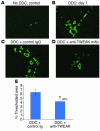
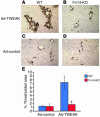
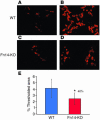
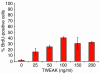
Progenitor ("oval") cell expansion accompanies many forms of liver injury, including alcohol toxicity and submassive parenchymal necrosis as well as experimental injury models featuring blocked hepatocyte replication. Oval cells can potentially become either hepatocytes or biliary epithelial cells and may be critical to liver regeneration, particularly when hepatocyte replication is impaired. The regulation of oval cell proliferation is incompletely understood. Herein we present evidence that a TNF family member called TWEAK (TNF-like weak inducer of apoptosis) stimulates oval cell proliferation in mouse liver through its receptor Fn14. TWEAK has no effect on mature hepatocytes and thus appears to be selective for oval cells. Transgenic mice overexpressing TWEAK in hepatocytes exhibit periportal oval cell hyperplasia. A similar phenotype was obtained in adult wild-type mice, but not Fn14-null mice, by administering TWEAK-expressing adenovirus. Oval cell expansion induced by 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) was significantly reduced in Fn14-null mice as well as in adult wild-type mice with a blocking anti-TWEAK mAb. Importantly, TWEAK stimulated the proliferation of an oval cell culture model. Finally, we show increased Fn14 expression in chronic hepatitis C and other human liver diseases relative to its expression in normal liver, which suggests a role for the TWEAK/Fn14 pathway in human liver injury. We conclude that TWEAK has a selective mitogenic effect for liver oval cells that distinguishes it from other previously described growth factors.
Figures



Similar articles
-
TWEAK/Fn14 pathway: a nonredundant role in intestinal damage in mice through a TWEAK/intestinal epithelial cell axis.Gastroenterology. 2009 Mar;136(3):912-23. doi: 10.1053/j.gastro.2008.11.017. Epub 2008 Nov 8. Gastroenterology. 2009. PMID: 19109961
-
Age-dependent effects of TWEAK/Fn14 receptor activation on neural progenitor cells.J Neurosci Res. 2007 Dec;85(16):3535-44. doi: 10.1002/jnr.21443. J Neurosci Res. 2007. PMID: 17803219
-
Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells.Hepatology. 2010 Jul;52(1):291-302. doi: 10.1002/hep.23663. Hepatology. 2010. PMID: 20578156
-
TWEAK/Fn14 pathway: an immunological switch for shaping tissue responses.Immunol Rev. 2011 Nov;244(1):99-114. doi: 10.1111/j.1600-065X.2011.01054.x. Immunol Rev. 2011. PMID: 22017434 Review.
-
TWEAKing tissue remodeling by a multifunctional cytokine: role of TWEAK/Fn14 pathway in health and disease.Cytokine. 2007 Oct;40(1):1-16. doi: 10.1016/j.cyto.2007.09.007. Epub 2007 Nov 5. Cytokine. 2007. PMID: 17981048 Review.
Cited by
-
Organotypic liver culture models: meeting current challenges in toxicity testing.Crit Rev Toxicol. 2012 Jul;42(6):501-48. doi: 10.3109/10408444.2012.682115. Epub 2012 May 15. Crit Rev Toxicol. 2012. PMID: 22582993 Free PMC article. Review.
-
Heterogeneity of hepatocyte dynamics restores liver architecture after chemical, physical or viral damage.Nat Commun. 2024 Feb 10;15(1):1247. doi: 10.1038/s41467-024-45439-0. Nat Commun. 2024. PMID: 38341404 Free PMC article.
-
Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling.Proc Natl Acad Sci U S A. 2013 Apr 16;110(16):6542-7. doi: 10.1073/pnas.1302168110. Epub 2013 Apr 1. Proc Natl Acad Sci U S A. 2013. PMID: 23576749 Free PMC article.
-
The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis.Proc Natl Acad Sci U S A. 2010 May 4;107(18):8248-53. doi: 10.1073/pnas.0912203107. Epub 2010 Apr 19. Proc Natl Acad Sci U S A. 2010. PMID: 20404163 Free PMC article.
-
Dynamics of cytokines predicts risk of hepatocellular carcinoma among chronic hepatitis C patients after viral eradication.World J Gastroenterol. 2022 Jan 7;28(1):140-153. doi: 10.3748/wjg.v28.i1.140. World J Gastroenterol. 2022. PMID: 35125824 Free PMC article.
References
-
- Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech. Dev. 2003;120:117–130. - PubMed
-
- Tan J, et al. Immunohistochemical evidence for hepatic progenitor cells in liver diseases. Liver. 2002;22:365–373. - PubMed
-
- Libbrecht L, Roskams T. Hepatic progenitor cells in human liver diseases. Semin. Cell Dev. Biol. 2002;13:389–396. - PubMed
-
- Roskams T. Progenitor cell involvement in cirrhotic human liver diseases: from controversy to consensus [review] J. Hepatol. 2003;39:431–434. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

