Inducible nitric oxide synthase (iNOS) expression in monocytes during acute Dengue Fever in patients and during in vitro infection
- PMID: 16109165
- PMCID: PMC1208887
- DOI: 10.1186/1471-2334-5-64
Inducible nitric oxide synthase (iNOS) expression in monocytes during acute Dengue Fever in patients and during in vitro infection
Abstract
Mononuclear phagocytes are considered to be main targets for Dengue Virus (DENV) replication. These cells are activated after infection, producing proinflammatory mediators, including tumour-necrosis factor-alpha, which has also been detected in vivo. Nitric oxide (NO), usually produced by activated mononuclear phagocytes, has antimicrobial and antiviral activities.
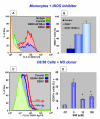
Methods: The expression of DENV antigens and inducible nitric oxide synthase (iNOS) in human blood isolated monocytes were analysed by flow cytometry using cells either from patients with acute Dengue Fever or after DENV-1 in vitro infection. DENV-1 susceptibility to iNOS inhibition and NO production was investigated using NG-methyl L-Arginine (NGMLA) as an iNOS inhibitor, which was added to DENV-1 infected human monocytes, and sodium nitroprussiate (SNP), a NO donor, added to infected C6/36 mosquito cell clone. Viral antigens after treatments were detected by flow cytometry analysis.
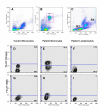
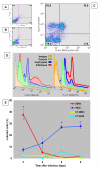
Results: INOS expression in activated monocytes was observed in 10 out of 21 patients with Dengue Fever and was absent in cells from ten healthy individuals. DENV antigens detected in 25 out of 35 patients, were observed early during in vitro infection (3 days), significantly diminished with time, indicating that virus replicated, however monocytes controlled the infection. On the other hand, the iNOS expression was detected at increasing frequency in in vitro infected monocytes from three to six days, exhibiting an inverse relationship to DENV antigen expression. We demonstrated that the detection of the DENV-1 antigen was enhanced during monocyte treatment with NGMLA. In the mosquito cell line C6/36, virus detection was significantly reduced in the presence of SNP, when compared to that of untreated cells.
Conclusion: This study is the first to reveal the activation of DENV infected monocytes based on induction of iNOS both in vivo and in vitro, as well as the susceptibility of DENV-1 to a NO production.
Figures


Similar articles
-
Enhancement by tumor necrosis factor alpha of dengue virus-induced endothelial cell production of reactive nitrogen and oxygen species is key to hemorrhage development.J Virol. 2008 Dec;82(24):12312-24. doi: 10.1128/JVI.00968-08. Epub 2008 Oct 8. J Virol. 2008. PMID: 18842737 Free PMC article.
-
Immunomodulating and antiviral activities of Uncaria tomentosa on human monocytes infected with Dengue Virus-2.Int Immunopharmacol. 2008 Mar;8(3):468-76. doi: 10.1016/j.intimp.2007.11.010. Epub 2007 Dec 26. Int Immunopharmacol. 2008. PMID: 18279801
-
Human TLR3 recognizes dengue virus and modulates viral replication in vitro.Cell Microbiol. 2009 Apr;11(4):604-15. doi: 10.1111/j.1462-5822.2008.01277.x. Epub 2009 Feb 4. Cell Microbiol. 2009. PMID: 19134117
-
Mechanisms of monocyte cell death triggered by dengue virus infection.Apoptosis. 2018 Dec;23(11-12):576-586. doi: 10.1007/s10495-018-1488-1. Apoptosis. 2018. PMID: 30267240 Review.
-
Endothelial dysfunction in dengue virus pathology.Rev Med Virol. 2015 Jan;25(1):50-67. doi: 10.1002/rmv.1818. Epub 2014 Nov 27. Rev Med Virol. 2015. PMID: 25430853 Review.
Cited by
-
Radical-Generating Activity, Phagocytosis, and Mechanical Properties of Four Phenotypes of Human Macrophages.Int J Mol Sci. 2024 Feb 3;25(3):1860. doi: 10.3390/ijms25031860. Int J Mol Sci. 2024. PMID: 38339139 Free PMC article.
-
Dengue virus infection induces upregulation of GRP78, which acts to chaperone viral antigen production.J Virol. 2009 Dec;83(24):12871-80. doi: 10.1128/JVI.01419-09. Epub 2009 Sep 30. J Virol. 2009. PMID: 19793816 Free PMC article.
-
Aedes aegypti saliva impairs M1-associated proinflammatory phenotype without promoting or affecting M2 polarization of murine macrophages.Parasit Vectors. 2019 May 16;12(1):239. doi: 10.1186/s13071-019-3487-7. Parasit Vectors. 2019. PMID: 31097013 Free PMC article.
-
Down-regulation of complement receptors on the surface of host monocyte even as in vitro complement pathway blocking interferes in dengue infection.PLoS One. 2014 Jul 25;9(7):e102014. doi: 10.1371/journal.pone.0102014. eCollection 2014. PLoS One. 2014. PMID: 25061945 Free PMC article.
-
A model of DENV-3 infection that recapitulates severe disease and highlights the importance of IFN-γ in host resistance to infection.PLoS Negl Trop Dis. 2012;6(5):e1663. doi: 10.1371/journal.pntd.0001663. Epub 2012 May 29. PLoS Negl Trop Dis. 2012. PMID: 22666512 Free PMC article.
References
-
- Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. - PubMed
-
- Monath TP. Pathobiology of the Flaviviruses. In: Schlesinger SSMJ, editor. he Togaviridae and Flaviviridae. New York , Plenun Press; 1986. pp. 375–440.
-
- Scott RM, Nisalak A, Cheamudon U, Seridhoranakul S, Nimmannitya S. Isolation of dengue viruses from peripheral blood leukocytes of patients with hemorrhagic fever. J Infect Dis. 1980;141:1–6. - PubMed
-
- Tassaneetrithep B, Burgess TH, Granelli-Piperno A, Trumpfheller C, Finke J, Sun W, Eller MA, Pattanapanyasat K, Sarasombath S, Birx DL, Steinman RM, Schlesinger S, Marovich MA. DC-SIGN (CD209) Mediates Dengue Virus Infection of Human Dendritic Cells. J Exp Med. 2003;197:823–829. doi: 10.1084/jem.20021840. - DOI - PMC - PubMed
-
- Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, Putvatana R, Louder MK, Filgueira L, Marovich MA, Wong HK, Blauvelt A, Murphy GS, Robb ML, Innes BL, Birx DL, Hayes CG, Frankel SS. Human skin Langerhans cells are targets of dengue virus infection. Nat Med. 2000;6:816–820. doi: 10.1038/77553. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

